
Risk Management Programme (RMP) Template
for Micro Abattoirs
You can use this RMP template if you:
• Slaughter and dress farmed mammals, ostriches or emus; and
• Have a low throughput; and
• Process meat for human consumption; and
• Operate a fixed or mobile micro abattoir premises; and
• Process meat for New Zealand and/or export to countries that do not
require Official Assurances.
How to complete and register the Risk Management Programme for Micro Abattoirs Page ii
This RMP template is issued by the Ministry for Primary Industries in accordance with
section 12 (3A) of the Animal Products Act 1999 for the purpose of making the
determination that the Risk Management Programme (RMP) Template for Micro Abattoirs
is valid and appropriate for the business of this kind described in the Statement of
Application.
Pages i to xvii are not part of the RMP.
Statement of Application
The application of the Risk Management Programme (RMP) Template for Micro Abattoirs is
limited to businesses that are operating a fixed or mobile micro abattoir premises for the
slaughtering and dressing of farmed mammals, ostriches or emus.
Dated at Wellington 12
th
day of December 2023.
Aaron Tangaroa
Manager Regulatory Delivery
Ministry for Primary Industries
(acting under delegated authority of the Director-General)
Contact for further information
Ministry for Primary Industries (MPI)
Animal Products
PO Box 2526
Wellington 6140
Email: animal.products@mpi.govt.nz

How to complete and register the Risk Management Programme for Micro Abattoirs Page iii
Contents
Contents iii
What this template covers v
How to Complete the Template vi
General vi
Part 1. Required Information viii
Part 2. Supporting Systems xiii
How to Register the RMP xvi
1.1 Complete the RMP template xvi
1.2 Complete the Application forms xvi
1.3 Apply for Registration xvi
1.4 Keeping the Registered RMP up-to-date xvii
Risk Management Programme for Micro Abattoirs 1
Part 1: Required Information 1
1.1 Identifying Information 1
1.2 Day-to-day Manager 1
1.3 Operator Name, Business Address and Contact Details 2
1.4 Scope of the RMP 3
1.5 Other Activities, Risk-based Measures or Operators 5
1.6 External Verification 6
1.7 RMP Document List 7
1.8 Authorisation of the RMP 10
Part 2: Supporting Systems 11
A. Document Control and Record Keeping 11
B. Personnel Health and Hygiene 14
C. Personnel Competencies and Training 18
D. Operator Verification 20
E. Design, Construction and Maintenance of Buildings, Mobile Premises, Facilities and
Equipment 24
F. Water 29
G. Cleaning and Sanitation 36
H. Receipt of Incoming Materials and Live Animals 39
I. Traceability, Inventory and Labelling 41
J. Calibration 43
K. Chemical Control 45
L. Pest Control 47
M. Non-conforming Product and Recall 49
N. Corrective Action 52
O. Storage 54
Part 3: Regulatory Limits and Hazard Analysis 1

How to complete and register the Risk Management Programme for Micro Abattoirs Page iv

How to complete and register the Risk Management Programme for Micro Abattoirs Page v
What this template covers
(1) This RMP template applies to the primary processing of farmed mammals, ostriches or
emus by the RMP operator.
(2) This RMP template applies to operators that are:
a) slaughtering and dressing farmed mammals, ostriches or emus; and
b) have a low throughput; and
c) process meat for human consumption; and
d) operate a fixed or mobile micro abattoir premises; and
e) process meat for New Zealand and/or export to countries that do not require
Official Assurances.
(3) A micro abattoir is not allowed to do any homekill or recreational catch activities under
section 70 of the Animal Products Act 1999.
(4) Micro abattoirs are not the same as dual operator butchers (DOB) who are able to
operate a retail butchery (secondary processing) that sells regulated animal products
and processes homekill or recreational catch (see Risk Management Programme
Template for Dual Operator Butchers).
(5) This RMP template does not apply to operators covered under a different RMP,
Regulated Control Scheme or a risk-based measure under the Food Act 2014 (e.g. Food
Control Plan or National Programme), or operators that process, transport and store:
a) other animal products; and
b) other food products; and
c) other non-food products.
(6) This RMP template has been developed based on New Zealand requirements only as
the scope of the template only covers product for New Zealand or for export to
countries where official assurances are not required.
(7) If your operations are not fully covered by this template, or you decide to deviate from
the requirements and procedures given in this template, you can do so by modifying
this template, or writing your own RMP. In most cases, these changes will need to be
evaluated by an MPI recognised RMP evaluator. If you decide to make changes to this
template after you have registered it, you will need to talk to your verifier first.

How to complete and register the Risk Management Programme for Micro Abattoirs Page vi
How to Complete the Template
General
(1) You need to provide complete and accurate information as the registered RMP is a
legally binding document that must be complied with. Everything written down needs
to accurately reflect or apply to your operation.
(2) You can complete this RMP template electronically as it is an editable PDF document,
or you can print it off and manually complete it. If you are manually completing your
RMP template, you must ensure that all information is clear and easy to read.
(3) The template should be completed by a person or group of people who have full
knowledge of the whole operation covered by the RMP.
(4) You need to read each section of this guidance while completing the template.
(5) You must provide the required information by entering information into the empty
boxes or blank lines; or ticking the appropriate answer or information.
(6) Your final RMP will be the completed RMP template (Part 1: Required Information,
Part 2: Supporting Systems and Part 3: Regulatory Limits and Hazard Analysis) and all
the additional documents you have written yourself and listed in the document list.
(7) You must comply with all the requirements and procedures in the final RMP, including
those in the supporting systems and all the additional documents you have written
yourself and listed in the document list.
(8) If you need to make changes to this template to better suit your operation, you can do
so by modifying this template or writing your own RMP. In most cases, these changes
will need to be evaluated by an MPI recognised RMP evaluator. If you decide to make
changes to this template after you have registered it, you will need to talk to your
verifier first.
(9) By complying with the requirements and procedures given in this template, you will be
meeting the requirements for the primary processing of animal products that are
specified in the current versions of:
Animal Products Act 1999
www.legislation.govt.nz/act/public/1999
/0093/latest/DLM33502.html
Animal Products
Regulations 2021
www.legislation.govt.nz/regulation/publi
c/2021/0400/latest/LMS520972.html

How to complete and register the Risk Management Programme for Micro Abattoirs Page vii
Animal Products Notice:
Production, Supply and
Processing
www.mpi.govt.nz/dmsdocument/50182
(10) You can refer to the Red Meat Code of Practice (www.mpi.govt.nz/food-
business/meat-game-processing-requirements/meat-game-codes-
practice-risk-management-programme-documents/) for additional useful
information.
(11) Where you need to develop additional procedures and forms, you can
use and adapt the examples of forms and procedures from the RMP
Operator Resource Toolkit (www.mpi.govt.nz/dmsdocument/26566).

How to complete and register the Risk Management Programme for Micro Abattoirs Page viii
Part 1. Required Information
1.1 Identifying Information
RMP ID – if you do not already have a RMP ID, you can nominate your own
identifier when you complete the AP4 Application form
(www.mpi.govt.nz/dmsdocument/71). Your identifier must be a number/letter
combination of at least 3 and no more than 10 characters, with at least one
character a number and no leading zeros.
If you have more than one RMP, assign a consecutive two digit number (01-99) to each new
RMP you have. Enter 01 if this is your first RMP.
For example: 100% ABC NZ Ltd could nominate an identifier of 100ABC/01 for their first
RMP.
If you don’t nominate an identifier, MPI will assign one for you. If the identifier you
nominate is not in the appropriate format, or is already in use, MPI will suggest an
alternative.
1.2 Day-to-day Manager
Day-to-day manager of the RMP – also referred to as the RMP Manager, you must nominate
a day-to-day manager who will be responsible for implementing the RMP and ensuring that
it is kept up-to-date. They will be the contact person for MPI and the verification agency
when dealing with matters relating to the RMP.
It is recommended that the position be given instead of the name of the day-to-day manager
to avoid the need for amending the template and notifying MPI when this person is
replaced. You may also wish to identify a deputy to the day-to-day manager.
Email – you must enter the email address that can be used to contact the Day-to-day
manager of the RMP.
Mobile phone number – you must enter a mobile phone number that can be used to contact
the day-to-day manager of the RMP. If there is no mobile number, a landline number may be
entered.
1.3 Operator Name, Business Address and Contact Details
NZBN – you must provide your NZBN here if you have one. If you want more
information about NZBNs, see www.nzbn.govt.nz.
Full Legal Name - if the business is a registered company, then you must use
the full legal name that matches the details given at the Companies Office exactly. If the
business is a partnership or a sole trader operation, then you must provide the name(s) of
the business owner(s)/partners.

How to complete and register the Risk Management Programme for Micro Abattoirs Page ix
Trading Name – you must fill this in if the name the business trades under (i.e. the name
used on a shop sign or letterhead) is different to the full legal name. If you don’t have a
trading name then you can leave this blank.
Physical address of premises (fixed premises) – you must give the street address of the
premises that the RMP applies to. If this RMP is for one business with multiple sites, include
the addresses for the additional sites as a separate, clearly named, attachment.
Main address of premises (mobile premises) – you must give the address of the location that
the mobile premise is parked when not in use.
Vehicle registration number (mobile premises) – you must give the vehicle registration
number of the mobile premises that the RMP applies to. If you have a mobile premise and
update your vehicle you need to update your RMP.
Postal address – if the postal address is different to the main address, you must give the
address any correspondence should be sent to, including the postcode.
Phone number – you must enter a phone number that can be used to contact the RMP
operator. Enter a phone number even if this is the same as the phone number under 1.2
Day-to-day Manager.
Mobile phone number – you must enter a mobile phone number that can be used to contact
the RMP operator. Enter a mobile phone number even if this is the same as the mobile
phone number under 1.2 Day-to-day Manager.
Email – you must enter the email address that can be used to contact the RMP operator.
Enter an email address even if this is the same as the email address under 1.2 Day-to-day
Manager.
1.4 Scope of the RMP
Physical Boundaries – you must include a site plan as part of the RMP. The site plan should
be labelled to make it clear it is part of the RMP. If this RMP covers more than one site, you
must attach a site plan for each site. Tick the box to indicate that you have a site plan and be
sure to attach it when submitting the RMP for registration.
For fixed premises, your site plan must show the buildings, facilities and
external surroundings included under your RMP. The different rooms or areas
within a building and the location of key pieces of processing and hygiene
equipment should also be shown in the diagram(s). The physical boundary of
the RMP will need to be clearly indicated on the site plan. Generally, the physical boundary
of a fixed premises is the legal boundary or the fence line of the property. Areas and facilities
within the boundary that are excluded in the RMP should also be clearly indicated on the
site plan. See the RMP Manual (www.mpi.govt.nz/dmsdocument/183) for an example.
For mobile premises, your site plan must show the layout of each vehicle, including storage
facilities, and the location of key pieces of processing and hygiene equipment on the site
plan. The physical boundaries of the RMP for a mobile premises are formed by the outer

How to complete and register the Risk Management Programme for Micro Abattoirs Page x
extremities of the mobile facility. Note: for a mobile premises, employee amenities do not
need to be located within the RMP premises.
Processing – tick the box(es) to indicate what processing your RMP covers. At the time of
registration, your operation must be capable of carrying out the processes that you indicate.
If you modify the template with additional processes these may need to be evaluated by an
MPI recognised RMP evaluator before your RMP can be registered with MPI.
1.5 Other Activities, Risk-based Measures or Operators
You must fill out this table if there are any products or activities that occur on the same
premises or within the physical boundaries of the RMP, but are not covered by this RMP
template because:
• they are covered under a different risk-based measure (e.g. an RMP, a Regulated
Control Scheme, a Food Control Plan, a National Programme or under the Wine Act
2003); or
• they are not covered under a different risk-based measure; or
• they are carried out by a different operator.
Examples of activities that you may wish to keep under the Food Act regime are: retail shop,
secondary processing activities only for the domestic market.
Under the Animal Products Act 1999, you are not allowed to use your micro abattoir
facility for any homekill or recreational catch activities.
Note: you must have procedures that make sure that these excluded activities are not a
source of contamination to any animal products processed or stored within the physical
boundaries of the RMP.
Fill out the table as appropriate, listing:
• each activity (including processing of other products) occurring within the RMP
physical boundary that is not covered by this RMP; and
• if the activity is covered under a different RMP, Regulated Control Scheme or risk-
based measure (if yes, say which one it is covered under and include the ID if there
is one); and
• how the activity is controlled, so operations are not adversely affected; and
• who is responsible for ensuring that the control measures are implemented and
effective; and
• who is responsible for resolving any issues that occur between this RMP, and the
other activity (use name or job title, include name of different operator if
applicable).

How to complete and register the Risk Management Programme for Micro Abattoirs Page xi
For example:
Activity Covered under a
Risk-Based Measure
(if Yes, include which
one and ID)
Control Measures
Responsibility
(Name or job title, include
name of different operator if
applicable)
Packaging of
eggs
RMP ID BUS111/01
Kept separate
from other
product and
activities
Packhouse Manager
Processing
animal feed for
sale
No
Kept separate
from other
product and
activities
Feed Mill Supervisor
Subcontract
space to store
packed paper
boxes
No
Boxes are only
allowed to be
stored in an
ambient storage
facility that is not
used by this RMP.
Storage facility has
a separate
entrance.
Lead Supervisor of the Pretty
Paper Company
If necessary, use extra pages and attach to the RMP.
1.6 External Verification
This section states that you authorise the contracted verifier to have freedom and access to
carry out verification activities. You must have a record of the name and contact details of
the verification agency and ensure that a letter has been received from the verification
agency confirming that they will verify your RMP. This letter must be provided to MPI when
applying for registration of your RMP. An electronic letter or email is fine.
The verifier must have access to any and all places, things and information that may
reasonably be needed to complete the verification (e.g. lab test results, non-conformances
and the corrective actions taken, etc.). You must tick the box to indicate that you have
contracted a verifier and have received and attached the letter from the verification agency
confirming that they will verify the RMP.
1.7 RMP Document List
Table 1: Documents from the RMP template. This gives the list of all the documents from the
RMP template that form part of your RMP. You must complete this table with the date
authorised for each document. This will be the date that the RMP is authorised (section 1.8).
How to complete and register the Risk Management Programme for Micro Abattoirs Page xii
Table 2: Procedures, programmes, water-use criteria. This table is for all the additional
documents that make up the rest of the RMP – these documents have been written by you.
You must fill in this section with the name of the document and include the name of the
person authorising the document and the date of authorisation for each of the procedures
and programmes you have written yourself or used from the RMP Operator
Resource Toolkit (www.mpi.govt.nz/dmsdocument/26566). If you have written
your own module(s), include them in this table.
Supporting systems of the RMP may require you to write procedures and
programmes covering good operating practice (GOP) and process control that are specific to
your operation and premises. Examples of the type of documents are: a cleaning
programme, cleaning schedules, calibration programme, inventory control procedures, etc.
The verifier will confirm the effectiveness of the RMP against these procedures and
programmes. You must ensure that all the written procedures and programmes apply to
your operation and that you comply with them.
These documents must be authorised by the day-to-day manager or a nominated person
and may be authorised individually and separately to the documents from the RMP template
(Table 1).
Each document must be re-authorised each time it is updated.
1.8 Authorisation of the RMP
The RMP must be authorised by either the day-to-day manager or a nominated person. Tick
the boxes to indicate which person is authorising. This person must sign, date and give their
job title.
If the person signing is a nominated person, check their name is on the list of nominated
persons referred to in the ‘Show’ section of Supporting System A. Document Control and
Record Keeping.
You must tick the boxes to confirm that you agree to the statements confirming that the
RMP is valid and appropriate for the activities it is intended to cover.
Each time you make a minor or significant amendment to the RMP, the RMP needs to be re-
authorised (signed and dated).
If you are electronically completing the RMP template and are unable to electronically sign,
then print this page, physically sign, and include a scan of the signed page when sending to
MPI.

How to complete and register the Risk Management Programme for Micro Abattoirs Page xiii
Part 2. Supporting Systems
The supporting systems in Part 2 describe the good operating practices and procedures
that you will comply with. They are part of your RMP and you will need to include them
when submitting your application.
You will need to:
a) read each supporting system thoroughly; and
b) ensure that everything in each supporting system applies to your operation and
that you will be able to comply with them; and
c) provide information suggested in some supporting systems that’s specific to your
operation by:
i) entering information into the empty boxes or blank lines; or
ii) ticking the appropriate answer or information.
d) ensure that you have written any procedures and programmes that might be
required and that these additional documents are listed in the Document List
(Section 1.7 in Part 1 of the template).
Your contracted verifier will verify the effectiveness of the RMP against the supporting
systems and the additional procedures and programmes you have written. It is a good idea
to store a copy of your procedures and programmes with your copy of the RMP.
Each supporting system is written in the Know/Do/Show format.
Know has general information about why this topic is important and gives ideas
for how you can comply with food law.
Do outlines what you must do to comply with the food safety laws.
Show gives examples of records which your verifier might want to see as
evidence that you’ve done something.
The pencil icon indicates that you need to:
• enter further details or tick boxes as appropriate (e.g. monitoring frequency for
compliance with procedures, etc.) directly in the supporting system; or
• write a procedure, programme or other document that covers the points listed in
the supporting system.

How to complete and register the Risk Management Programme for Micro Abattoirs Page xiv
You can find help on writing procedures, programmes or other documents in
the Red Meat Code of Practice (www.mpi.govt.nz/food-business/meat-game-
processing-requirements/meat-game-codes-practice-risk-management-
programme-documents/).
You can find example forms and procedures in the RMP Operator Resource
Toolkit (www.mpi.govt.nz/dmsdocument/26566).
The document icon indicates that you need to keep a record of something.
Monitoring for Operator Verification
What is this?
Many of the supporting systems have a section called ‘Monitoring for Operator Verification’,
where you write in a frequency for checking that you are meeting the procedures.
Making sure that procedures are being followed is part of the ‘Operator Verification’. We
have added the ‘Monitoring for Operator Verification’ sections to help you meet these
requirements.
What timeframes should I put?
Operator Verification of procedures needs to be done at least once a year. For most
supporting systems, reviewing every 1-3 months would be appropriate. However, for an
activity that happens daily, a monthly review may be too infrequent. For an activity that
happens every month (or less often), 3 monthly might be too frequent.
Choose timeframes that are both appropriate for what you are reviewing and are
achievable.
Additional guidance for the Water supporting system
Use of water for a mobile premises
Mobile premises can use their own supply of water that meets the water-use plan (e.g.
carried in suitable containers).
Mobile premises can only use water from the property they visit if that water is also under a
water-use plan (i.e. the water is being supplied from another RMP premises who has
completed this assessment).

How to complete and register the Risk Management Programme for Micro Abattoirs Page xv
You can also write your own water-use plan to include the use of town-supply water (that is
not further treated) from any property you visit.
Town supply water
If you are using town supply water without treating it yourself, whether you need to develop
water-use criteria and perform initial water testing depends on if you have a reason to
believe the town supply water will not meet the E. coli and turbidity requirements (the
standard requirements for all water).
Generally, you can assume that town supply water will meet the standard requirements. In
this situation, the completed Water supporting system is your water use plan. You do not
need to create water-use criteria, do initial testing or routine monitoring.
If you have a reason to believe that the town supply water will NOT meet the standard
requirements, then you need to document the reason you are unsure, and you will need to
develop water-use criteria and do initial water testing. Depending on the results of the initial
testing, you may need to do routine monitoring as well.
Own-source water
If you are using own-source water, especially on mobile premises, you will need to develop
water-use criteria and do initial water testing. Depending on the results of the initial testing,
you may need to do routine monitoring as well.
You can complete the Own-source water checklist and template water-use
plan (www.mpi.govt.nz/dmsdocument/56140). When this is completed, this,
combined with the Water supporting system, will be your water use plan and
will include the water-use criteria.
The Own-source water checklist and template water-use plan doesn’t cover all possible
sources of water. If your source is not covered (e.g.: sourced from another RMP operator or
water where additional treatment is applied by you), you will have to write your own water-
use plan and water-use criteria. You could use the checklist and the Water supporting
system to help you do this. You will need to meet the water requirements in
Chapter C of the Animal Products Notice: Production, Supply and Processing
(www.mpi.govt.nz/dmsdocument/50182).

How to complete and register the Risk Management Programme for Micro Abattoirs Page xvi
How to Register the RMP
1.1 Complete the RMP template
You must complete all parts of the RMP template and write any additional procedures or
other documents that you need.
If changes have been made to the template
If your operations are not fully covered by this template, or you decide to deviate from the
requirements and procedures given in this template, you will need to modify this template
with additional information or write your own RMP. In most cases, these will need to be
evaluated by an MPI recognised RMP evaluator.
If you decide to modify the template after you have registered it, talk to your verifier first.
1.2 Complete the Application forms
Fill in both of these application forms:
• Application Form AP4: Registration of Risk Management
Programme (www.mpi.govt.nz/dmsdocument/71)
• Application Form AP49: Processing Categories Tables
(www.mpi.govt.nz/dmsdocument/4562)
1.3 Apply for Registration
To apply for registration of your RMP, send the following information to MPI Approvals
• completed RMP template, which is Part 1: Required Information and Part 2:
Supporting Systems.
– check you have added the name and date of issue for each document you have
created yourself to 1.7 RMP Document List,
• completed Application Form AP4: Registration of Risk Management Programme
– check you have included all additional documents required by the AP4 form,
• completed Application Form AP49: Processing Categories Tables
MPI may ask for clarification or further information on any part of the RMP. There may be an
additional assessment fee charged for the time of the MPI assessor so it is advisable to
complete the RMP template and application forms as best as you can. The RMP will be
registered once MPI is satisfied with the RMP and all fees are paid.
How to complete and register the Risk Management Programme for Micro Abattoirs Page xvii
1.4 Keeping the Registered RMP up-to-date
Updates to information held in the template can be made. Amendments to contact details
such as emails, phone numbers or postal addresses can be made by emailing the information
to be changed to approvals@mpi.govt.nz.
Amendments to other details such as the trading name and the name of the
day to day manager will be a minor amendment and an AP50: Registration of a
Minor Amendment (www.mpi.govt.nz/document-vault/4567) form must be
completed and emailed to [email protected].
When making any amendment to an RMP, you have to determine whether the amendment
is considered significant or minor. Detailed guidance on RMP amendments is given in the
RMP Manual. Appendix G of the manual provides examples of significant and minor
amendments. You can also consult your RMP verifier when deciding whether an amendment
is significant or minor.
Other minor amendments may require notification to MPI (you will need to submit an AP50:
Registration of a Minor Amendment (www.mpi.govt.nz/document-vault/4567) form).
Significant amendments are to be submitted using the AP6: Risk Management
Programme Amendment Registration (www.mpi.govt.nz/dmsdocument/4573).
If the amendment relates to an activity outside the scope of the RMP
template, the amended RMP will require evaluation.
All amendments made to the RMP should be recorded in an Amendment
Register (www.mpi.govt.nz/dmsdocument/26566). A sample register is
included in this link to the RMP Operator Resource Toolkit.
Pages i to xvii are not part of the RMP and DO NOT need to be
submitted to MPI
The RMP starts on the next page, page 1
Risk Management Programme for Micro Abattoirs – Required Information Page 1
Risk Management Programme for Micro
Abattoirs
Part 1: Required Information
Please complete the tables as required.
1.1 Identifying Information
RMP ID
1.2 Day-to-day Manager
Name, position or
designation of the Day-to-day
Manager of the RMP
Email
In entering this email, I consent to being sent information and notifications electronically.
Mobile phone number

Risk Management Programme for Micro Abattoirs – Required Information Page 2
1.3 Operator Name, Business Address and Contact Details
NZBN
Full Legal Name
Trading Name, if any (if
different from legal name)
Physical address (for fixed
premises)
or
Main location address for
mobile premises)
Vehicle Registration
Number (for mobile
premises only)
Postal address including
postcode (if different from
the physical address)
Phone number
Mobile phone number
Email

Risk Management Programme for Micro Abattoirs – Required Information Page 3
1.4 Scope of the RMP
Type of premises
Type of premises covered by the RMP:
☐
Fixed premises
The layout and RMP physical boundaries of the
fixed premises are shown on the attached site
plan.
☐
Mobile premises
The layout of the mobile premises (i.e. the
vehicle) is shown on the attached site plan.
This RMP applies to the following animal species:
The species covered by the RMP:
☐
Cattle (excluding bobby calves)
☐
Buffalo
☐
Deer
☐
Sheep
☐
Pig
☐
Goat
☐
Alpaca
☐
Llama
☐
Horse
☐
Rabbit
☐
Emu
☐
Ostrich
Risk Management Programme for Micro Abattoirs – Required Information Page 4
Processes
The RMP covers the following processes
(Tick all applicable processes)
☐
Receiving of live animals
☐
Ante-mortem examination
☐
Slaughter
☐
Post-mortem examination
☐
Dressing
☐
Collection of offal and co-products
☐
Cooling of carcasses and offal

Risk Management Programme for Micro Abattoirs – Required Information Page 5
1.5 Other Activities, Risk-based Measures or Operators
These activities occur within the physical or mobile boundaries of the RMP, but are excluded from the RMP and:
• they are covered under a different risk-based measure (e.g: an RMP, a Regulated Control Scheme, a Food Control Plan, a National Programme or under the Wine Act 2003);
or
• they are not covered under a different risk-based measure; or
• they are carried out by a different operator.
Procedures are in place for ensuring that these products are not a source of contamination to any products that are stored in the premises.
Activity
Covered under a Risk-Based
Measure
(if Yes, include which one and ID)
Control Measures
Responsibility
(Name or job title, include name of
different operator if applicable)
Transport of carcasses
Consider safe approaches to loading carcasses on to
transport to ensure this process doesn’t interfere with
food safety.

Risk Management Programme for Micro Abattoirs – Required Information Page 6
1.6 External Verification
(1) I give my contracted risk management programme verifier access to any and all
places, things and information that may reasonably be needed to complete the
verification, including:
a) freedom to access premises, places, or facilities covered by a risk
management programme; and
b) access to documents, records, and information that relate to a risk
management programme; and
c) access to things (including containers and packages) that are used in
connection with processing animal material, animal products, non-animal
product foods and non-food animal products under a risk management
programme; and
d) access to animal material, animal product, equipment, packages, containers,
and other associated things used in processing animal material, animal
product, non-animal product foods, and non-food animal products under a
risk management programme (noting that the verifier may identify and mark
any of those things); and
e) such freedom to examine and take samples (for the purpose of analysis or
retention) of animal material, animal product, non-animal product foods,
non-food animal products, or any other outputs, substance, or associated
thing which has been, is, or may be used in contact with, or in the vicinity of
animal material, animal product, non-animal product foods, or non-food
animal products being produced or processed under a risk management
programme.
(2) I will provide my contracted risk management programme verifier with any
reasonable assistance requested.
(3) By way of explanation, in the case of a significant risk to the fitness for intended
purpose of animal product or suitability of animal material for processing, a
recognised risk management programme verifier may recommend to an Animal
Product Officer that the officer exercises their powers of interruption of operations
under section 89 of the APA which (in
the case only of the powers under section 89(b)
and (c)) may be exercised by the Animal Product Officer over the phone if he or she
considers that appropriate.
☐
A letter (e.g. hardcopy or electronic confirmation such as an email) has been
received from the verification agency confirming they will verify the risk
management programme at all sites covered by this risk management programme.

Risk Management Programme for Micro Abattoirs – Required Information Page 7
1.7 RMP Document List
Table 1: Documents from the RMP template
The date authorised will be the same as the date Section 1.9 is signed.
Title Date Authorised
Part 1: Required Information
Part 2: Supporting Systems
Part 3: Regulatory Limits and Hazard Analysis

Risk Management Programme for Micro Abattoirs – Required Information Page 8
Table 2: Additional documents written by the operator
These additional documents include: procedures; programmes; site plan; list of nominated
persons; water checklist; amendment record etc.
These documents must be authorised by the day-to-day manager or a nominated person
and may be authorised individually and separately to the documents from the RMP template
(Table 1).
Each document must be re-authorised each time it is updated.
Updating a document you have written yourself might be a minor or significant amendment.
Title Authorisation
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:

Risk Management Programme for Micro Abattoirs – Required Information Page 9
Title Authorisation
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:
Name:
Date:

Risk Management Programme for Micro Abattoirs – Required Information Page 10
1.8 Authorisation of the RMP
I confirm that:
☐
All of the documents listed in Section 1.7 are appropriate for my operation.
☐
All building, facilities and equipment necessary to implement the RMP are
available and ready to operate.
☐
The RMP, including all relevant legislation incorporated into the RMP, will be
implemented as written.
☐
☐
The documents from the RMP template, including all Supporting Systems have
been authorised by:
The day-to-day manager of the programme
or
A nominated person
Signature
Title:
_________________________________________________________________
Date
The RMP must be re-authorised (signed and dated) each time a minor or significant
amendment is made to the documents from the RMP template (i.e. section 1.7 Table 1).

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 11
Part 2: Supporting Systems
A. Document Control and Record Keeping
Useful things to know
• To ensure all RMP documents are authorised, controlled, kept up-to-date, and
stored properly.
• To ensure records are generated and stored properly.
Rules you must follow
Document control
• Every document that forms part of this RMP is dated and authorised (see RMP
Document List (Tables 1 & 2) by:
− the Day-to-day manager; or
− a nominated person.
• All current RMP documents and their date of authorisation are listed in the RMP
Document List (Tables 1 & 2).
• All RMP documents are:
− able to be clearly read; and
− indicate their version or date of authorisation.
• Details of all amendments to the RMP, including minor and significant
amendments, are recorded in an Amendment Register. (The RMP Manual
(www.mpi.govt.nz/dmsdocument/183) has guidance on determining if an
amendment is minor or significant.)
• The most recent amendments made in a document are identified by highlighting
or marking the amended part(s).
• Current versions of RMP documents are readily available, in hard copy or
electronic form, to persons with key responsibilities in operating the RMP.
Record keeping
• A list of the nominated people (who can authorise documents, as per above
section) is kept.
• All records identified in the RMP are clear and readable.
• All paper and electronic RMP records (e.g. monitoring, corrective action,
verification and validation records) include:
− the date and, where appropriate, the time of the activity or observation;
− an accurate description of the results of the activity or observation; and
− the identity of the person(s) who performed the activity (i.e. initials or
signature of the person completing the record).
• Any alteration made to a record is made in a way that allows the original entry to
remain readable (i.e. erasures or the use of correction fluid or tape or other
material to cover the original entry is not allowed) and is initialled by the person
making the alteration.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 12
• The following records are kept for at least 4 years:
− a copy of every supplier statement received from suppliers;
− records that provide the following information for each mob of animals:
• date and time of arrival
• supplier (name in clear wording or in code)
• number of animals
• class of animals
• any marks, brands, or other distinguishing features on the animals, if
the holding facility contains animals from more than one supplier
• information to determine where the animals from the mob are being
held.
Accessibility and retention of all RMP documents and records, including archived
documents
• One copy of all RMP documents and all records, including those that are
obsolete/out-dated/previous versions, are:
− retained for 4 years, or for the duration of the shelf-life of the product
(whichever is longest); and
− stored in a location where they are protected from damage, deterioration or
loss.
• Any validation information is:
− kept for the life of the process or activity; or
− until the process is revalidated and new records are created (then the old
validation information is archived and retained for 4 years)
• All electronic RMP documents and records are backed up regularly.
• All RMP documents and records, including archived documents, are able to be
made available to the RMP verifier or any person authorised by MPI, within 2
working days of a request being made.
Amendments
• All amended parts of the RMP are replaced with the current versions without
unnecessary delay after authorisation.
• An amendment register, which includes the following information, is maintained
by the RMP operator:
− document and specific part being amended;
− details of amendment;
− reason for amendment;
− date of change; and
− person approving the amendment.
• Any alterations on records are made alongside the original entry and initialled by
the person altering the record.
Monitoring for Operator Verification
• Compliance with these procedures is checked at least ______________ by the
responsible person.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 13
Things to show your verifier
• Document list.
• List of nominated persons (if any)
• Obsolete documents and documents are filed.
• Records are complete and available upon request (e.g. In the RMP Operator
Resource Toolkit Amendment Register).
• Supporting System and process control records (including monitoring, corrective
action and verification records).
• Record forms.
• All records generated while operating the RMP.
Examples of these forms can be found in the RMP Operator Resource
Toolkit
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 14
B. Personnel Health and Hygiene
Useful things to know
• To ensure that all personnel are medically fit to perform their duties, and that
they comply with good hygienic practices to prevent or minimise the
contamination of product.
• Personnel include all workers, staff, contractors providing services and visitors.
Rules you must follow
Induction and ongoing supervision of personnel
• New personnel are informed of their job description, health requirements, and
hygienic practices and procedures before starting work.
Health and sickness policy
• The Day-to-day Manager ensures that all personnel understand and comply with
the health and sickness requirements discussed in this section.
• All personnel (including visitors and contractors) are required to inform the Day-
to-day Manager or another responsible person if they are suffering from any of
the health conditions listed in Table B.1 below.
• Personnel suffering from a health condition or illness listed in Table B.1 should
not carry out tasks where animal products may be affected.
• There are procedures to deal with any event where animal products are
contaminated (e.g. blood or vomit on product). Refer to M. Non-conforming
Product and Recall and N. Corrective Action
Table B.1. Health conditions
Condition or illness
Diarrhoea or vomiting due to gastroenteritis or other infectious diseases
including norovirus and rotovirus.
(May also include illnesses involving E. coli, Salmonella spp., Shigella spp.,
Campylobacter, Yersinia, Cryptosporidium, Giardia, and Vibrio cholerae)
Acute respiratory infection
Hepatitis A
Skin infection (e.g. boils, sores, infected wounds, etc.)
Protective Clothing
• Ensure that protective clothing is visibly clean at the start of each day’s operation.
• Ensure protective clothing and footwear is:
− maintained in a hygienic condition;
−
made of readily cleanable materials;

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 15
− cleaned or changed if it becomes a source of contamination during
processing; and
− stored in a manner that protects it from contamination.
• Ensure disposable or damaged protective clothing and footwear is:
− discarded after use, when damaged (e.g. torn), or if it cannot be effectively
cleaned when required during use; or
− repaired.
• Everyday clothes are not worn over protective clothing.
• Reusable aprons are cleaned and sanitised at least daily.
• Disposable aprons and gloves are discarded after use, or when torn, damaged or
contaminated.
• Waterproof protective clothing (e.g. aprons, gloves) is not worn outside
processing areas.
• Protective clothing is not worn in areas outside the premises where there is
potential for them to be exposed to contaminants.
Gloves
• If used, re-useable gloves are cleaned and sanitised periodically during the day’s
operations, and at the end of the day’s operation or shift using the appropriate
method below.
− Cut-resistant gloves are soaked in appropriate sanitiser overnight and rinse
with warm water prior to use; or
− Chain mesh gloves are hosed with high pressure hot water to remove visible
soil, soak in appropriate sanitiser (20-25%) for no less than 15 minutes, soak
in hot water for no less than 15 minutes, rinse with high pressure hot water,
and hang to dry.
Washing of hands and arms
• All personnel thoroughly wash hands and exposed parts of the arms with
approved liquid soap and water, and then dry them using disposable paper
towels (or a suitable alternative):
− after using the toilet;
− after handling or coming into contact with waste and contaminated surfaces
or material; and
− after contaminating the hand from coughing, sneezing or blowing the nose.
Note: If clean water is not readily available for hand washing in certain areas,
alternative options for sanitising personnel hands may be considered.
Jewellery and other personal items
• Personnel in processing areas are not permitted to wear watches, rings and
other jewellery except for plain wedding bands (e.g. no stone). Plain wedding
bands may be worn only when they cannot be easily dislodged, and they can be
effectively cleaned in the same manner as hands.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 16
• False eyelashes, false fingernails, nail polish and other nail art are not permitted
in processing areas, unless they can be worn under gloves (without piercing the
gloves).
• Devices (e.g. medic alerts) and cultural jewellery (e.g. taonga necklace, wedding
jewellery) may be worn in processing areas provided they cannot be easily
dislodged, and they are able to be securely worn under clothing or gloves.
• Personnel are not permitted to take personal items (e.g. cigarettes, small loose
items) into processing areas that may result in contamination of products and
processing areas.
Note: A processing area in this part means areas where materials and products
are exposed, or food contact surfaces may be contaminated.
Visitors and contractors
• All visitors and contractors are required to report to the responsible person on
arrival.
• Visitors and contractors who enter processing or storage areas are required to
confirm that to the best of their knowledge they have no medical condition that
may pose a risk to food safety. Records of this should be kept (e.g. signed
declaration or logbook).
• If a visitor or contractor is visibly ill, the responsible person can deny them access
to processing or storage areas.
• Prior to entering the processing or storage areas visitors and contractors should
wear clean protective clothing and footwear that are provided or approved by
the Day-to-day Manager.
• Where appropriate, visitors and contractors are supervised by assigned staff
while within the premises. The assigned staff are responsible for ensuring that
visitors and contractors follow hygienic practices and procedures.
Hygienic practices
• Personnel behave in a manner that prevents the contamination and deterioration
of product and the environment.
• Eating, drinking, smoking, vaping or spitting are not allowed inside the processing
areas.
Visitors and contractors
• Where appropriate, visitors and contractors are supervised by assigned staff
while within the premises.
Monitoring for Operator Verification
• Compliance with these procedures is checked at least ______________ by the
responsible person.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 17
Things to show your verifier
• A record of all employee illnesses and any medical certificates e.g. Staff Sickness
form.
• Completed register for injuries (or similar).
• Completed training records e.g. Personnel Training Form.
• Any problems detected and any corrective actions taken. Refer to N. Corrective
Action.
Examples of these forms can be found in the RMP Operator Resource
Toolkit

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 18
C. Personnel Competencies and Training
Useful things to know
• To ensure that all personnel have the necessary knowledge, skills, and training to
perform their assigned tasks in a competent and hygienic manner, including
relevant experience and ongoing refresher training in hygienic slaughter and
dressing of animals.
• For additional useful information, refer to Operator Verification Guidance
(www.mpi.govt.nz/dmsdocument/40898)
Rules you must follow
Competencies of key RMP personnel
• All personnel (other than the Day-to-day Manager) who have been nominated to
authorise the documents that form this RMP are identified (either by position, or
by name and position).
• Personnel responsible for the following key tasks are identified (either by
position, or by name and position).
− process control,
− operator verification,
− corrective action,
− undertaking recalls,
− monitoring at Critical Control Points,
• Personnel performing key tasks have the following competencies:
− knowledge and skills in executing the particular task; and
− an overall understanding of the area they are working in.
• The skills or competencies are documented on the Personnel Training Form.
Day-to day Manager
• The Day-to-day Manager is responsible for:
− ensuring proper implementation of documented RMP programmes and
procedures, including monitoring of processes and taking corrective actions
for any non-compliances;
− keeping RMP documents up-to-date;
− verifying the effectiveness of the RMP;
− communicating with the RMP verifier, as needed; and
− ensuring all personnel are adequately trained.
• The Day-to-day Manager has a good understanding of the documented RMP,
including legal requirements and supporting systems.
• The Day-to-day Manager is identified (either by position, or by name and
position) in the RMP.
• The RMP is amended if the Day-to-day Manager changes. Refer to D. Operator
Verification.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 19
Induction and supervision
• New personnel are informed of the following before they start working:
− the company’s health and sickness requirements;
− hygienic practices;
− movement of personnel and materials;
− cleaning and sanitation;
− handling of chemicals;
− hygienic handling of materials and products; and
− operational procedures for their assigned tasks.
• Ongoing supervision and/or skills maintenance is provided to ensure that
personnel are adequately trained in their specific tasks, and in hygienic practices
and procedures.
• The training programme includes:
− identification of skills and competencies required for key roles;
− training schedules (including refresher training); and
− training records of personnel.
Visitors and contractors
• Visitors and contractors report to a responsible person on arrival at the premises
or mobile premise location. Where appropriate, visitors and contractors are
supervised by assigned staff while within the premises.
• It is the responsibility of the assigned staff to ensure that hygienic practices and
procedures are followed by the visitor or contractor.
• Visitors and contractors are not allowed to handle materials or product in
processing and packing areas unless they have complied with all hygiene
requirements for food handlers.
Monitoring for Operator Verification
• Compliance with these procedures is checked at least ______________ by the
responsible person.
Things to show your verifier
• Competencies identified for key roles e.g. job descriptions, training matrix
• Training and qualification certificates.
• Completed e.g. Training Programme
• Completed e.g. Personnel Training Form.
Examples of these forms can be found in the RMP Operator Resource Toolkit.
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 20
D. Operator Verification
Useful things to know
• Operator verification is a system of internal checks that confirms the
effectiveness of the RMP by:
− checking procedures are being followed (as noted at the end of most
supporting systems)
− corrective actions and preventative actions are taken
− reporting requirements are met
− other operational requirements are met (i.e. notification, amendments)
− establishing frequencies for checks, what is being checked, how and by
whom
− ensuring checks (including periodic monitoring and internal audits) are done
at the required frequencies.
• For additional useful information, refer to Operator Verification Guidance
(www.mpi.govt.nz/dmsdocument/40898)
Rules you must follow
Operator verification
• The Day-to-day Manager ensures that the RMP is effective by making sure that
the following checks are done:
− all activities that contribute to operator verification are transparent and
traceable, and undertaken by suitably skilled persons nominated by the Day-
to-day Manager
− persons carrying out operator verification activities are (if possible)
independent of the process or operation monitoring and corrective action
activities being verified. They are familiar with the contents of the RMP,
including its expected outcomes.
Table D.1: Operator verification activities and frequencies
Activity
Details
Frequency
Record checks
• Collect all records and check they
are complete, correctly filled out,
and that all results are acceptable or
the
appropriate corrective action has
been taken and documented.
• Review to identify any trends, new
hazards or recurring problems.
• When completed.
Personnel
supervision
• Ensure that all personnel are
following correct practices and
procedures.
• As required.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 21
Review of RMP
• Read through the RMP and amend it
where necessary.
• Perform a reality check to ensure
documented procedures are
followed.
• Test your recall plan by conducting
mock recalls for meat.
• Significant amendments will be
evaluated and registered.
• At least annually.
• When procedures
or premises
change.
• When RMP is not
working
effectively.
Internal audits
• Internal audits are an example of operator verification.
• Internal audits are about performing checks to ensure:
− the RMP is up to date and covers all activities.
− there are no uncontrolled food safety risks.
− the products are fit for intended purpose.
− staff know, understand and are correctly applying the procedures in the
RMP.
• The person responsible for undertaking internal audits has:
− a good understanding of the operations, processes and GOP covered by the
RMP; and
− a good understanding of the regulatory requirements.
• The person performing the internal audit does a reality check, which includes
observing staff, equipment and premises to make sure that:
− staff are following hygienic procedures and operating procedures;
− staff are following operating parameters (e.g.: temperatures); and
− hygienic status of the premises, internal and external environment and
equipment is maintained.
• A sample of records are checked during the internal audit to make sure the
correct things are being recorded.
• All findings from previous internal audits and external verification visits are
followed up to make sure they have been fixed.
• Any new issues found during the internal audit are identified and corrected.
Records are kept of this.
• When ongoing or recurring non-compliances occur, the following actions are
taken:
− investigate to determine possible causes of non-compliance;
− take appropriate corrective actions to regain control and prevent recurrence
of the problem;
− increase surveillance of the system; and
− review the RMP or the relevant Supporting Systems and make necessary
changes.
• Indications that the RMP or parts of it are not working effectively include:
− repeated non-compliance or out of specification product test results;
− customer complaints;
−
multiple or repeated issues raised by the RMP verifier; or
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 22
− unacceptable outcomes from external verification visits.
RMP review
• The RMP is reviewed annually to check for any significant changes, such as
changes to equipment, facilities, personnel positions, RMP verifier, etc.
Significant Amendments
• After any significant amendment to the RMP has come into effect, all parts of the
RMP that may be affected by the amendment are checked to ensure they are still
effective and properly implemented.
HACCP plan review
• The HACCP plan is reviewed annually to check for any changes (e.g. to process
flow, inputs or outputs, new hazards, etc.).
Recording issues and findings
• The completed audits are recorded e.g. in the Annual Internal Audit Check
Sheets.
• Issues or findings requiring action and corrective action taken, are recorded e.g.
in the Corrective Action Register.
Notification
• The Day-to-day Manager will send an email to Food.Compliance@mpi.govt.nz
and their RMP verifier notifying of any product that is recalled because it is not or
may not be fit for its intended purpose.
letter to the Manager, Approvals Operations, MPI, PO Box 2526, Wellington 6140
notifying of any (it is recommended to inform your RMP verifier):
− change to the name, position or designation of the Day-to-day Manager of
the RMP; and
− change in RMP verifier.
99 66 (for biological hazards only) notifying of any emerging, new or exotic
biological hazards or new chemical hazards that have been discovered.
• The Day-to-day Manager will contact the recognised RMP verification agency
without unnecessary delay on discovering:
− significant concerns about the fitness for intended purpose of any product;
− that the cumulative effect of minor amendments necessitates the
registration of a significant amendment to the RMP;
− that the RMP is no longer effective;
− that the premises are no longer suitable for their use;
− that anything within the physical boundaries of the RMP is used for
additional purposes or by other operators, and the RMP has not adequately
considered relevant hazards or other risk factors;
−
merging two or more registered RMPs; or
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 23
− splitting a registered RMP into two or more RMPs.
Who’s responsible?
Record the name or position of the person(s) responsible for undertaking/organising
Operator Verifications __________________________________________________
_________________________________________________________________
_________________________________________________________________
Things to show your verifier
• Any information or evidence relating to operator verification activities (e.g.
temperature readings).
• Internal audit documentation.
• RMP verifier audit reports.
• Completed e.g. Annual Internal Audit Check Sheets.
• Any problems detected and any corrective action taken. Refer to N. Corrective
Action.
• Copies of any emails or letters sent to MPI or the RMP verifying agency.
Examples of these forms can be found in the RMP Operator Resource Toolkit.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 24
E. Design, Construction and Maintenance of Buildings, Mobile
Premises, Facilities and Equipment
Useful things to know
• To ensure that all buildings, mobile premises, facilities and equipment are
designed, constructed, installed and operated in a manner that prevents or
minimises contamination of product, packaging, other inputs, equipment and the
processing environment.
• The requirements and procedures in this supporting system apply to both fixed
and mobile premises, unless specifically stated otherwise.
Rules you must follow
Buildings and facilities
• Internal structures of buildings, including floors, ceilings and walls, are designed
and constructed to:
− minimise contamination and cross-contamination of products;
− be durable and capable of withstanding repeated exposure to normal
cleaning and sanitising;
− resist corrosion;
− minimise the entrance and harbourage of pests;
− minimise the accumulation of condensation;
− minimise the entry of environmental contaminants; and
− be free from cracks and crevices that may harbour contaminants.
• Facilities are available and kept in a satisfactory condition for:
− hygienic processing, packing and storage of products;
− storage of chemicals, cleaning compounds and other materials;
− storage and reticulation of water;
− cleaning and sanitation of facilities and equipment;
− personnel hygiene (e.g. toilets, hand washing units, showering facilities,
storage lockers); and
− drainage and disposal of wastes.
• Facility and equipment layout (e.g. working space) allows for good hygienic
practices, access by personnel and effective cleaning.
• Essential services (e.g. lighting, ventilation, process gases) are sourced, used and
maintained in a way that enables effective operation.
• Lighting is sufficient to enable effective operations.
• All site and building entrances are clearly marked to deter unauthorised entry.
• Buildings and facilities are managed in a way that protects product, packaging
and other inputs from adulteration.
• Vehicle access and parking areas are designed and constructed to prevent
contamination of processing areas.
• Any glass, including light fixtures, is safety glass, or otherwise protected to
prevent contamination of the products, materials or packaging.
•
Windows are sealed.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 25
Mobile premises
• Mobile premises will follow the requirements for buildings (as appropriate).
• A mobile premises when operating its RMP, is located in an area that is:
− tidy and well maintained;
− adequately drained and not prone to flooding; and
− away from areas and activities that may impact on the hygienic operation of
the RMP and cause contamination of products and the processing
environment.
Animal holding facilities
• Animal holding facilities where animals are held prior to slaughter
need to comply with animal welfare requirements under the Animal
Welfare Act 1999
(www.legislation.govt.nz/act/public/1999/0142/latest/DLM49664),
and the Code of the Code of Welfare Commercial Slaughter 2018
(www. https://www.mpi.govt.nz/dmsdocument/46018)
• The facilities need to:
− effectively contain animals,
− facilitate ante-mortem examination,
− allow normal mobility and an easy flow of animals from the holding facility to
the slaughter facility, and
− for fixed premises, allow effective cleaning and effective drainage of water
and liquid waste.
• For fixed premises, separate holding facilities that meet the same requirements
given above, are provided for the holding of suspect animals and for the post-
mortem examination of animals found to be dead or dying.
Equipment
• Equipment that comes into contact with products is designed, constructed,
installed and operated in a manner that:
− ensures the effective performance of the intended task;
− facilitates cleaning and sanitising; and
− minimises contamination of the product.
• Suitable cleaning equipment (maintained in a hygienic condition) is available for
cleaning and sanitising of equipment and facilities. Refer to G. Cleaning and
Sanitation.
• Any equipment designed to cool products is operated within its design and
capacity, and consistently delivers the required temperature.
• Measuring equipment (whether stand alone or forming part of a piece of
equipment), has the accuracy, precision, and conditions of use appropriate to the
task performed. Refer to J. Calibration.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 26
Slaughter and dressing facilities
• Animal restraining and stunning equipment needs to comply
with the requirements of the Code of Welfare Commercial
Slaughter 2018 (www.mpi.govt.nz/dmsdocument/4601).
• Where a moving chain system is used, chain stopping devices
are provided to facilitate hygienic processing and carcass examination, and
ensure safe operations.
• Rails or other carcass elevating devices, or cradles are provided to ensure that
carcasses do not come into contact with contaminated equipment and surfaces
during processing.
• Facilities for washing waterproof protective clothing (e.g. boots, aprons, gloves)
are provided.
• Adequate space and facilities are provided for post-mortem examination so that
all parts of an animal can be examined effectively.
• Facilities or designated areas for retaining carcasses or carcass parts are
provided.
• Facilities are provided for secure holding and disposal of condemned material.
• Facilities and equipment used for condemned materials are properly identified.
• Chillers (and freezers, if available)
− are capable of reducing product temperatures to the required temperature
within the prescribed time, and maintain product temperatures at or below
the required temperature
− have the capacity appropriate for the volume of products likely to be
processed or held in the refrigeration facility at any one time; and
− are fitted with a temperature measuring device located where accurate air
temperature readings can be made (e.g. warmest location of the
refrigeration unit) and which can be monitored by the operator.
Repairs and maintenance
• Alterations, repairs and maintenance are done when necessary to ensure that
facilities and equipment are in a suitable condition.
• Processing stops if the facilities and equipment are in a condition that will affect
the product and make it not suitable for its intended use.
• Procedures set out:
− which areas and equipment are regularly checked for any issues that could
lead to damage or deterioration of product or packaging, and when or how
often checking is done;
− any other checking or inspection for maintenance that must be done;
− how assessment of the impact that maintenance work will have on
processing is done; and
− what corrective actions must be taken if product or packaging is affected by
maintenance.
• All alterations, repairs and maintenance work on facilities and equipment
(including refrigeration and freezing units) are done in a manner that minimises

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 27
the exposure of product or packaging to hazards introduced by this work.
Corrective actions are taken if needed. Refer to N. Corrective Action.
• If any maintenance activity affects the suitability for intended use of the product,
then action is taken to stop more product being affected, including (if required)
stopping processing.
• Before use of facilities and equipment, a suitably skilled person checks that:
− maintenance is sufficiently complete so that when processing re-starts,
product will not be adversely affected; and
− areas and surfaces have been appropriately cleaned and, where appropriate,
sanitised; and
− if processing had stopped during the work, the area has been returned to a
suitable state for processing to re-start.
Changes
• MPI will be notified if there are plans to make major alterations to facilities or
equipment which may impact on the product(s) (this can be a significant
amendment to the RMP).
Refrigerated and frozen facilities and equipment
• Refrigerated and frozen facilities are designed, constructed and equipped to
ensure that the specified preservation temperatures are maintained throughout
storage.
• If installed, equipment for the control and accurate monitoring of temperatures
and any other required refrigeration or frozen parameters (e.g. humidity, air-
flow, etc.) are operated at an appropriate frequency, at all times that
refrigeration and frozen facilities are in use.
• Temperature measuring devices are located to measure the internal temperature
of the storage facility at the warmest point and are calibrated.
Note: Temperature measuring devices for critical measurements should be
calibrated at a frequency necessary for maintaining its required accuracy.
Operators should refer to the equipment supplier’s recommendation for guidance.
Recording issues and findings
• Issues or findings requiring action and the corrective actions that are taken are
recorded e.g. in the Repairs and Maintenance Register.
Monitoring for Operator Verification
• Compliance with these procedures is checked at least ______________ by the
responsible person.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 28
Things to show your verifier
• Completed e.g. Repairs and Maintenance Register, Maintenance Schedule,
Maintenance Form.
• Any equipment specifications, manufacturers’ or suppliers’ instructions (e.g. any
specifications or manuals related to refrigeration units).
• Any building reports.
• Any problems detected and any corrective action taken. Refer to N. Corrective
Action.
• Calibration records.
Examples of these forms can be found in the RMP Operator Resource
Toolkit.
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 29
F. Water
Useful things to know
• To ensure that water is fit for its intended purpose at the point of use and
maintains the fitness for intended purpose of product.
• Where the water used can’t affect the animal products, this Water Supporting
System doesn’t apply (e.g. if water used for toilets is from a separate source).
• The requirements and procedures in this supporting system apply to both fixed
and mobile premises, unless specifically stated otherwise.
Rules you must follow
Water supply
• The source of water used within the premises is (tick all applicable):
☐ town supply water (a reticulated water supply that provides drinking
water to the public; no further treatment may be applied by the RMP
operator)
☐ own-source water (water other than town-supply water, or reused or
recovered water; e.g. water sourced from a bore, river, stream, roof; water
sourced from another RMP operator; water where additional treatment is
applied by this operator)
☐ reused or recovered water
Water use
• Water is used for:
− cleaning of facilities and equipment;
− personal hygiene activities;
− production of steam;
− use in washing equipment; and
−
other operational activities where water comes into direct or indirect contact
with any product.
Design and management of reticulation system
• The on-site water reticulation system is designed, installed and operated in a
manner that ensure water is delivered for the purpose for which it is intended;
and:
− minimises dead ends and backflow; and
− prevents the contamination of water and unintentional mixing between
water intended for different purposes.
• Water lines, including flexible hoses, in processing areas that contain water of
different standards (such as water that is unsuitable for direct or indirect contact
with animal material or animal product) must be labelled or otherwise identified.
• Water pipes, storage tanks and other parts of the reticulation system are
maintained in good condition.
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 30
• The reticulation system is flushed (i.e. taps are opened at point-of-use to allow a
significant flow of water to occur) when water is not used for an extended period
and after any repairs to the system, to ensure that stagnant water, rust, scale or
other material is flushed out of the system.
Standard requirements for all water
Table F.1: Standard requirements for all water
Measurement Criteria
E. coli
Not Detected per 100 ml
Turbidity
Must not exceed 5 NTU (Nephelometric turbidity units)
Water use criteria
Table F.2: Water-use criteria
Water source Water-use criteria
Town supply water
(without additional
treatment)
☐ Water-use criteria is not required (assume the water
meets Table F.1 Standard Requirements for All Water)
or
☐ Water-use criteria is required (there are reasons to
believe the water will not meet Table F.1 Standard
Requirements for All Water)
Own-source water
Water-use criteria is required.
Reused or recovered
water
Water-use criteria is required.
• If water-use criteria is required under Table F.2, water-use criteria is developed
e.g. using Own-source water checklist and template water-use plan
• The water-use criteria must:
− reflect the source of the water and the purpose for which it is used; and
− be developed by a suitably skilled person; and
− be based on an assessment of any chemical, biological, physical, or
radiological hazards or other risk factors.
• The suitably skilled person who developed the water use criteria is
_________________________________________________________________
Name or position. Complete only if water use criteria is required.
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 31
Sampling and Testing
Table F.3: Initial testing and routine monitoring
Water source Initial testing Routine monitoring
Town supply water
(without additional
treatment)
☐ Initial testing is not
required (assume the
water meets Table F.1
Standard Requirements
for All Water)
or
No routine monitoring is
required.
☐
Initial testing is
required (there are
reasons to believe the
water will not meet Table
F.1 Standard
Requirements for All
Water)
☐
No routine monitoring is
required as
initial testing meets
Table F.1 Standard
Requirements for All Water.
or
☐ Routine monitoring is done
as per Table F.4 Frequency of
Testing and any additional
testing required under the
water-use criteria.
Own-source water
Initial testing is required.
☐
No routine monitoring is
required as
initial testing meets
Table F.1 Standard
Requirements for All Water
and the water-
use criteria does
not require additional testing.
or
☐ Routine monitoring as per
Table F.4 Frequency of Testing
and any additional testing
required under the water-use
criteria.
Reused or recovered
water
Initial testing is required.
Routine monitoring as per
Table F.4 Frequency of Testing
and any additional testing
required under the water-use
criteria.
• If testing is required under Table F.3, initial testing is done before processing
begins.
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 32
• Samples are obtained and handled in a manner that ensures they are:
− representative of the water being tested; and
− appropriate to the type of test.
• Water testing to ensure that the water meets the standard water requirements
(see Table F.1: Standard Requirements for All Water) and any relevant water-use
criteria is performed by a laboratory accredited for those tests.
The accredited laboratory used is
__________________________________________________________________
Complete only if water testing is required.
• Water testing to monitor parameters relating to water treatment (e.g. chlorine,
pH, turbidity) is performed by a suitably skilled person using methods
documented in the water-use plan, and if appropriate, calibrated equipment.
The suitably skilled person(s) who perform the water testing are
_________________________________________________________________
Name or position. Complete only if water testing relating to water treatment is
required.
Table F.4: Frequency of testing
Average daily use
while processing
Microbiologic
al testing (E.
coli or total
coliforms)
Turbidity
testing
pH testing (for
water
chlorinated
on site)
Chlorine testing
(for water
chlorinated on
site)
<100 m
3
/day and
product packaged
at all times
1 per 6
months
1 per 6
months
1 per 6
months
Daily when staff
present and
premises
operating
100 - 1000 m
3
/day
and product
packaged at all
times
1 per 3
months
1 per 3
months
1 per 3
months
Daily when staff
present and
premises
operating
<2000 m
3
/day
1 per month
1 per month
1 per month
Daily when staff
present and
premises
operating
2000 – 10 000
m
3
/day
1 per 2 weeks
1 per 2
weeks
1 per 2 weeks
Daily when staff
present and
premises
operating
>10 000 m
3
/day
1 per week
1 per 6
months
1 per 6
months
Daily when staff
present and

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 33
premises
operating
Additional requirements for water treated by the operator
• When water is treated by the operator (e.g. chlorination, boiling, filtration, UV
treatment, etc.), the water-use plan includes:
− information about the treatment applied, including the type of treatment,
operating procedures and parameters, monitoring procedures, and any
acceptable limits;
− a water sampling and testing programme for verifying the effectiveness of
the specific water treatment applied (frequency as indicated in Table F.4
Frequency of Testing or as necessary for the effective monitoring of any
specific water treatment applied); and
− corrective action procedures when the water is found to be unsatisfactory
based on the results of any test done.
• All equipment used for treating water is installed, maintained and operated as
per the manufacturer’s instructions.
• The water treatment system is developed and operated by a suitably skilled
person.
Reassessment
• The water supply is reassessed:
− at least once every 3 years;
− prior to using a new supply of water (that is, the supply changes, or a new
supply is added); and
− within 1 month after any change (that may adversely affect the water’s
fitness for intended purpose) to the water source (excluding town supply
water); the environment in or around the water source (excluding town
supply water); the reticulation system; the intended purpose of the water;
and any aspect of the treatment system (if relevant).
• The reassessment is documented.
• Reassessment is done by considering the information that has gone into the
water-use plan, water-use criteria and updating.
• When using town supply water,
the 3 yearly or new supply of water reassessment
also considers whether need to change from ‘assume the water meets Table F.1
Standard Requirements for All Water’ to ‘there are reasons to believe the water
will not meet Table F.1 Standard Requirements for All Water’ (or the reverse).
Corrective Actions
• When water is not fit for purpose, corrective action is taken (see Table F.5
Examples of Corrective Actions).
• Affected products are managed as non-conforming product, refer to M. Non-
conforming Product and Recall.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 34
Table F.5: Examples of corrective actions
Example Scenarios Actions
The town water supplier advises that
the water is not fit for drinking
without additional treatment
The following actions are taken as
appropriate to the scenario:
Immediate control and investigation of
problem
• all operations requiring the use of water
are stopped;
• the cause of the problem is investigated;
and
• appropriate corrective actions are taken
to rectify the problem (e.g. through
further treatment).
Disposition or handling of affected
products and equipment
• any affected product intended for
human consumption is not used for that
purpose unless assessment by a suitably
skilled person indicates that an
alternative action will render the product
safe and suitable for human
consumption;
• any affected product intended for
human consumption may be regraded
for animal consumption (e.g. petfood,
stockfood, etc.) when the product meets
the applicable requirements;
• any affected food contact surfaces are
cleaned and sanitised prior to reuse; and
• any affected packaging materials and
containers that cannot be effectively
cleaned and sanitised, are not used for
packaging of any product.
Records of the assessment and corrective
actions taken are kept.
Water fails to comply with any of
the requirements of the water
management plan (including
corrective actions) and there are no
other means in the RMP to ensure
the water meets the original standard
at the point of use
For water supplied by another RMP
or FCP, the other RMP or FCP
operator advises the operator that
the water does not meet the relevant
water standard
Water supply is contaminated by
non-complying water
The RMP operator or Day-to-day
Manager has reason to believe that
the water is not fit for use and there
are no procedures included in the
RMP to ensure the water is fit for
purpose at the point of use

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 35
Things to show your verifier
• Water reticulation plan (e.g. site plan).
• Own-source water checklist (if applicable) e.g. Own-source water checklist and
template water-use plan
• Results of water testing (if applicable).
• Results of ongoing monitoring of any water treatment activities (if applicable).
• Water use criteria (if applicable).
• Documentation of reassessment.
• Any problems informed of or detected (e.g. notification from water supplier,
failure of water treatment plant).
• Any problems detected and any corrective action taken. Refer to N. Corrective
Action.
• Completed e.g. Internal Audit reports.
Examples of these forms can be found in the RMP Operator Resource
Toolkit.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 36
G. Cleaning and Sanitation
Useful things to know
• To ensure the effective cleaning and sanitation of premises, facilities and
equipment to prevent or minimise the contamination of products.
• Cleaning means the physical removal of material from surfaces, including fat,
protein and mineral deposits.
• Sanitising means the inactivation of bacteria on cleaned surfaces and the
protection of cleaned surfaces until processing starts.
Rules you must follow
Cleaning
• There is a cleaning programme or schedule that covers all the different areas of
the premises and contains the following information:
− area, facility and/or equipment to be cleaned;
− procedures for cleaning the area, facility and/or equipment;
− type or method of cleaning;
− chemicals that are used;
− frequency of cleaning;
− frequency of cleaning checks or inspections;
− person/position responsible for cleaning;
− what corrective actions to take; and
− records to be kept.
• All relevant equipment, containers and food-contact surfaces (e.g. tables, cutting
boards, hooks, knives, saws, bins) are cleaned and sanitised regularly during the
day.
• Cleaning activities are carried out in a way that minimises contamination of
products, previously cleaned areas, etc.
Equipment for cleaning
• Cleaning equipment does not contaminate carcass or packaging.
• Cleaning equipment is:
− used for cleaning purposes only;
− stored in a hygienic manner when not in use; and
− maintained in a good state of repair.
• Hose nozzles are kept off the floor at all times to prevent back-siphonage and
contamination of staff hands
Wet cleaning
− All equipment and product contact surfaces that are wet cleaned should be
free from residues and moisture before processing restarts.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 37
Chemicals
• Cleaning compounds are used in accordance with the procedures given in K.
Chemical Control.
• Chemicals used for cleaning and maintenance are handled and used:
− according to the directions of the manufacturer; and
− in a manner that minimises contamination of product.
Management of cleaning chemical contamination
• If equipment or product contact surfaces are (or are suspected to be)
contaminated with residues, the affected equipment and product contact
surfaces are cleaned and sanitised prior to reuse.
• If carcass or packaging is (or is suspected to be) contaminated with residues:
− affected products are managed as non-conforming product, refer to M. Non-
conforming Product and Recall;
− affected packaging materials are either washed and sanitised (where
practicable) prior to use or are not used for packing any product for human
or animal consumption.
Collection and removal of solid waste
• Waste (including waste water) is not allowed to accumulate in or around
processing areas.
• Solid wastes are:
− collected in clearly identified waste containers, which are cleaned when
necessary;
− collected using clearly identified equipment that is stored in an identified
area when not in use;
− kept under controlled conditions to ensure that they are not mistakenly or
fraudulently released as suitable for processing for human consumption; and
− regularly disposed of in a way that ensures that they do not become a source
of contamination to products, the work area and the processing or storage
environment.
• Outside waste bins (where used) are covered, maintained in a tidy condition, and
collected regularly so that they do not attract pests and create objectionable
odours.
• To ensure animal materials are processed and stored in a hygienic manner, which
prevents or minimises contamination and proliferation of hazards on carcasses
and other animal material; and
• The requirements and procedures discussed in this supporting system apply to
both fixed and mobile premises, unless specifically stated otherwise.
Cleaning inspection
• Cleaning checks or inspections are undertaken on a regular basis to:
− ensure compliance with the cleaning and sanitation programme; and
−
check the effectiveness of cleaning.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 38
• Checks of facilities and equipment are done prior to use (including after
maintenance) to ensure that operations begin only after sanitation requirements
have been met:
− all observations made during the check are recorded.
• If a problem is found, then:
− the problem and the corrective actions are recorded;
− the source of the contamination is fixed (immediately if there is a food safety
risk); and
− the frequency of cleaning and sanitising is reviewed.
Monitoring for Operator Verification
• Compliance with these procedures and the effectiveness of cleaning is checked at
least ______________ by the responsible person. For poor results, increase the
frequency of checks. Once good results are achieved, decrease the frequency of
checks back to standard.
Things to show your verifier
• Cleaning schedules and procedures.
• Cleaning and pre-operational records, forms or check sheets.
• Completed e.g. Chemical Register.
• Any problems detected and any corrective action taken. Refer to N. Corrective
Action.
Examples of these forms can be found in the RMP Operator Resource
Toolkit.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 39
H. Receipt of Incoming Materials and Live Animals
Useful things to know
• To ensure only live animals eligible for processing for human consumption are
accepted and slaughtered at the premises.
• The requirements and procedures discussed in this supporting system apply to
both fixed and mobile premises, unless specifically stated otherwise.
• To ensure that packaging materials are fit for intended purpose, and that meat
products remain fit for intended purpose during packing.
• All packaging and product contact materials (e.g. carcass wrap, legging paper,
plastic liners and offal bags) are suitable for food contact use.
• Re-packing means making / breaking up small consignments and placing into
cartons for individual customer consignments.
Rules you must follow
Check supplier statements
• The RMP Manager, or person in charge, checks that a correctly completed supplier
statement, using the MPI approved Animal Status Declaration (ASD) form, is
provided at the time of presentation of a farmed animal for slaughter.
• An animal is not accepted for processing, if:
− the ASD is absent or incomplete;
− there are reasonable grounds to suspect that the information in the supplier
statement is fraudulent – in which case the RMP Manager informs MPI or the
verifier within 1 working day of becoming aware of the potential fraud; or
− the supplier statement or any poison use statement does not confirm the
status of the animal material as suitable for processing.
• For fixed premises, when the supplier statement for an animal(s) is absent or
incomplete, the live animal may be temporarily held in a holding pen in order to
give the supplier an opportunity to provide a completed or a replacement supplier
statement.
• The following records are kept for at least 4 years:
− a copy of every supplier statement received from suppliers; and
− records that provide the following information for each mob of animals:
• date and time of arrival;
• supplier (name in clear wording or in code);
• number of animals;
• class of animals;
• any marks, brands, or other distinguishing features on the animals, if
the holding facility contains animals from more than one supplier; and
• information to determine where the animals from the mob are being
held.
• National Animal Identification and Tracing systems are in place for cattle and deer
and records are kept of these.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 40
Receipt of incoming materials
• All packaging and product contact materials are suitable for food contact use.
• Opened cartons of packaging are re-closed and covered during storage to prevent
dust contamination.
• Packaging materials and other food contact materials are:
− checked on delivery to ensure they are fit for their intended use (i.e. clean,
undamaged) and properly labelled;
− protected against contamination or damage during storage; and
− kept separate from chemicals and other hazardous materials.
Packing and Re-packing
• Packing or re-packing of products is done under hygienic conditions, in a manner
that ensures that any product not enclosed in packaging is protected from
contamination and maintains its fitness for intended purpose by:
− the area being clean;
− personnel being suitably clothed;
− ensuring that products designed for re-packing are being managed via the
inventory system; and
− ensuring that all re-packaged products are appropriately labelled.
• All products remain identifiable at all times.
• Damaged packaging is disposed of appropriately.
Use of packaging materials
• Packaging is clean and undamaged at point of use.
• Dirty or damaged packaging is disposed of appropriately.
• Packaging materials adequately protect the product.
Monitoring for Operator Verification
• Compliance with these procedures is checked at least ______________ by the
responsible person.
Things to show your verifier
• Records of products under the RMP (e.g. consignment notes, incoming product
checks etc).
• Any problems detected and corrective action taken. Refer to N. Corrective Action.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 41
I. Traceability, Inventory and Labelling
Useful things to know
• To ensure that meat is correctly identified at storage and dispatch for inventory
control purposes and to allow for traceability in the event of a recall.
Rules you must follow
Inventory control
• Inventories are maintained for all meat.
• Non-conforming materials and products are clearly identified and the reasons for
non-conformance are in the inventory.
• All outgoing products are clearly labelled and accompanied by appropriate
documentation to ensure their traceability. Refer to Labelling of Transportation
Outers below.
Traceability
• A tracking system is maintained that:
− allows for the identification of all animal product throughout the entire
production chain even once it leaves your abattoir.
− can trace animal material and animal product from the supplier to the
operator; and from the operator to the next recipient in the supply chain
(other than the final consumer).
• Upon request by MPI, traceability information can be provided within 24 hours.
• All outgoing products are clearly labelled/tagged and accompanied by
appropriate documentation to ensure traceability.
Records
• Records include, as appropriate:
− name and address of suppliers of animals;
− Animal Declaration Status and NAIT details for cattle and deer;
− details about the supplied item, including the batch number, quantity and
delivery date;
− supplier status of any approved suppliers;
− an inventory system (either electronic or hard copy) that allows finished
products to be traced;
− load in and load out checks; and
− the name and address of the person or company who the batch of products
are delivered to.
Monitoring for Operator Verification
• Compliance with these procedures is checked at least ______________ by the
responsible person.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 42
Things to show your verifier
• Records showing products received (e.g. consignment notes, etc.).
• Any re-labelling or re-packing done.
• An inventory system (electronic or hard copy) that allows finished products to be
traced.
• Copies of labels.
• Any problems detected and corrective action taken. Refer to N. Corrective
Action.
Examples of these forms can be found in the RMP Operator Resource Toolkit.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 43
J. Calibration
Useful things to know
• To ensure that measuring equipment that is used to carry out critical
measurement functions as intended.
• Critical measurements are those that monitor controls for significant hazards.
• Critical measurements can include:
− chilling temperatures; and
− freezing temperatures.
• If your measurement is not providing a critical measurement, then you do not
need to follow this supporting system, however it is recommended to do so.
Rules you must follow
Measuring Equipment
• Measuring equipment (such as temperature probes, etc) that is used to provide
critical measurements are:
− accurate and fit for their intended use;
− calibrated regularly against a reference standard (i.e. shows traceability of
calibration to a national or international standard of measurement); or
− if no such standard exists, calibrated by a suitably skilled person using a
documented method (e.g. those in the RMP Resource Toolkit); and
− are uniquely identified (e.g. by using serial numbers, indelible tags or other
permanent means of identification) to enable traceability of the calibrations
to a reference standard and to identify the calibration status.
• A calibration programme is in place that covers the following:
− how to calibrate each piece of measuring equipment that requires
calibration;
− whether each piece of measuring equipment is used for taking critical
measurements or not;
− minimum frequencies of calibration for each piece of measuring equipment
used to provide critical measurements, or used as reference standards;
− safeguards for prevention of unauthorised adjustments to the calibration of
measuring equipment; and
− the corrective actions to be taken when a measuring device is damaged or
provides inconsistent or inaccurate readings and identification and
disposition of any product produced when the device was out of order.
Receipt of critical measuring equipment (new or repaired)
• Calibration certificates are requested from suppliers of critical measuring
equipment.
Chiller or freezer gauges
• Cool room temperature gauges are checked by placing another thermometer in
the cool room, next to the existing probe, for about 10 minutes then comparing
against the cool room temperature gauge.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 44
• Checks of automatic temperature devices are recorded on the Automatic
Temperature Recorder Checks Form.
Faulty equipment
• Equipment that is faulty or inaccurate is not used. It is repaired and recalibrated
or replaced as soon as possible.
Monitoring for Operator Verification
• Compliance with these procedures is checked at least ______________ by the
responsible person.
Things to show your verifier
• Calibration certificates and other calibration records.
• Identification, location and calibration status of equipment.
• Completed e.g. Calibration Form.
• Any problems detected and corrective action taken. Refer to N. Corrective
Action.
Examples of these forms can be found in the RMP Operator Resource Toolkit.
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 45
K. Chemical Control
Useful things to know
• To ensure the proper use and storage of chemicals to prevent or minimise the
contamination of carcass, packaging, processing aids, equipment and the
processing and storage environment.
• Chemicals include maintenance compounds used for cleaning, sanitation, pest
control and repair and maintenance of equipment.
Rules you must follow
Chemicals (including maintenance compounds)
• There are procedures for the storage, handling and use of chemicals.
• Only MPI approved maintenance compounds, as listed in the MPI Approved
Maintenance Compounds (Non-dairy) Register
(www.foodsafety.govt.nz/registers-lists/maintenance-
compounds/index.htm), are used:
− during processing operations;
− in the maintenance of processing areas; and
− on equipment.
• A list (register) of all chemicals used and held on the premises is kept and up-to-
date.
Storage of chemicals
• Chemicals are stored in a designated area, away from carcasses and processing
aids for example Carcass Spray.
• Chemicals are clearly labelled. If it is an approved maintenance compound, must
be labelled with the name as it appears on the list of approved maintenance
compounds.
• Chemicals are kept in sealed containers when not in use.
Use of chemicals
• Maintenance compounds are used according to the directions of the
manufacturer and the conditions of the approval.
• Directions for use (such as the detergent/sanitiser to be used in an area or on a
piece of equipment, their concentration, application method and contact time
required) are readily available to the user (e.g. given on the label, product
information data sheets, etc.).
• Chemicals are handled and used by, or under, the supervision of suitably trained
or experienced personnel.
• All containers or implements used for measuring or pouring of hazardous
chemicals are labelled ‘For Chemicals Only’ (or similar), to ensure they are not
used for any other purpose.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 46
• Products and unprotected packaging are removed or kept protected (e.g.
covered) prior to the use of chemicals (e.g. insecticide sprays) to prevent
contamination.
• Equipment and other food contact surfaces are cleaned by thorough washing
after exposure to chemicals that are not approved for food contact.
Handling and disposal of chemicals
• Empty chemical containers are disposed of and are not re-used in a way that may
contaminate product.
• When contamination by a hazardous chemical occurs, the following actions are
carried out:
− affected food contact surfaces are cleaned and sanitised prior to reuse;
− affected products are considered unfit for human or animal consumption
and are disposed of as per M. Non-conforming Product and Recall; and
− affected packaging that cannot be effectively cleaned and sanitised is
disposed of properly.
Monitoring for Operator Verification
• Compliance with these procedures is checked at least ______________ by the
responsible person.
Things to show your verifier
• Approved chemicals used (e.g. Chemical Register, consignment notes, etc.).
• Any problems detected and corrective action taken. Refer to N. Corrective
Action.
Examples of these forms can be found in the RMP Operator Resource Toolkit.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 47
L. Pest Control
Useful things to know
• To ensure effective control of pests so as to prevent or minimise the
contamination of product, packaging, other inputs, equipment and the
processing environment. Pests include rodents, wild birds, insects and cats.
Rules you must follow
Responsibility
• Pest control and monitoring activities within the RMP premises is carried out by
(tick applicable box):
☐ the RMP operator
☐ a contracted pest control person or agency
• Where pest control and monitoring activities are contracted out, the Day-to-day
Manager, prior to signing the contract or services agreement, ensures that:
− the person or agency to be contracted is competent to perform the task and
is familiar with the requirements of this Supporting System; and
− the written contract or services agreement clearly defines the services to be
provided by the contracted person or agency.
Controls to prevent entry of pests
• Buildings and facilities are designed and constructed in a manner that minimises
the entry of pests.
• External doors that are not screened are kept closed when not in use.
• Pets, excluding farm dogs in the yards, are not permitted anywhere within the
premises.
• Drains are fitted with screens.
• For fixed premises consider fitting insect screens on windows and external doors
that are kept open during operations.
Controls to prevent infestation of pests
• Buildings, external surroundings and waste bins are kept clean and tidy to
prevent potential breeding sites. Waste bins are regularly collected and emptied.
• Buildings are kept in good repair and condition to prevent pest access and
potential breeding sites.
• Regular inspections of the premises, including external surroundings, are carried
out to check for evidence of possible infestation.
• If present, electric insect traps are not installed above unprotected product or
packaging. The insect tray is emptied when necessary and any UV light bulb
changed as recommended by the manufacturer.
Use of pesticides (e.g. fly sprays, rat baits, etc.) and pest traps
• Pesticides are approved, handled, used and stored according to chemical control
requirements. Refer to K. Chemical Control.
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 48
• Pesticides are used according to the manufacturer’s directions and the MPI
conditions of the approval. Refer to the MPI website Approved
Maintenance Compounds
(www.mpi.govt.nz/processing/maintenance-compounds/non-
dairy-maintenance-compounds/).
• Bait stations are:
− identified (e.g. numbered); and
− located and installed so they cannot contaminate product or packaging (it is
preferred that bait stations are external, and not placed in manufacturing
areas).
• A record is kept of bait station locations.
• Bait stations and traps are checked at least ______________ for evidence of pest
activity (e.g. nibbled bait, bait missing, droppings, etc.) and to confirm they are in
good working order.
• Increased monitoring and appropriate corrective actions are undertaken when
increased rodent activity is observed.
• Any pests are regularly removed from the pest stations and the bait replaced if
required. This is recorded on a Vermin Control Register.
Handling and disposition
• Where there is evidence of contamination by pests, the following actions are
carried out:
− affected food contact surfaces are cleaned and sanitised prior to reuse;
− affected products are managed as non-conforming product, refer to M. Non-
conforming Product and Recall;
− affected packaging is either washed and sanitised (where practicable) prior
to use or is not used for packing any product for human or animal
consumption.
Monitoring for Operator Verification
• Compliance with these procedures is checked at least ______________by the
responsible person.
Things to show your verifier
• A contract or service agreement with the contracted pest control person or
agency, if applicable.
• A record of the location of the bait stations (may be shown on site plan used to
show physical boundaries).
• A record of all Approved Maintenance Compounds (pesticides) used (including
name, amount and point of use) (Refer to K. Chemical Control).
• Completed e.g. Vermin Control Register of pest sighting and monitoring.
• Any problems detected and corrective action taken. Refer to N.
Corrective Action.
Examples of these forms can be found in the RMP Operator Resource
Toolkit.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 49
M. Non-conforming Product and Recall
Useful things to know
• To ensure the correct handling and disposition of carcass and products, including
the recall of products from distribution and sale.
Rules you must follow
Non-conforming product
• Non-conforming product is any product that:
− has not been processed in accordance with relevant regulatory
requirements, and procedures written in the RMP, or
− is not safe or suitable for its intended use.
Suspected non-conforming product
• Product that is suspected of being non-conforming is managed as if it is non-
conforming.
• A suitably skilled person may determine that product that is suspected of being
non-conforming is actually conforming by considering various factors, such as:
− what the incident was
− the risk of breaching a regulatory or operator defined limit
− has the limit actually been breached (may require testing to be done)
− discussion with verifier
• If product is determined to be conforming, records are kept that cover:
− identification of the suspected non-conforming product; and
− a description of the event or circumstance that led to the product being
suspected non-conforming; and
− the justification for the product being determined as conforming.
Managing non-conforming product
• Non-conforming products are handled and stored in a manner that prevents:
− contamination and deterioration of other products or inputs; and
− contamination of the processing and storage environment that could lead to
contamination of other products or inputs.
• Non-conforming products are:
− clearly identified;
− separated from other products;
− identified in inventory (unavailable for load-out); and
− held until disposition is determined by a suitably skilled person or, in certain
cases, by the RMP verifier or MPI.
• The RMP verifier is notified as soon as possible when there is significant concern
about fitness for intended purpose of any products.
• The disposition of any non-conforming product is determined by a suitably skilled
person considering various factors, such as:
−
product safety and suitability;

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 50
− the amount of product affected;
− options for disposing of the product (such as reprocessing, downgrading, or
disposing of it as waste);
− whether the products have been released for distribution or not; and
− any instructions from MPI or the RMP verifier.
• Records are kept that cover:
− identification of the affected animal material or animal product;
− where the product was distributed to and
− a description of the event or circumstance that led to the product being non-
conforming; and
− the products disposal, including confirmation of actual disposal.
Unforeseen Events
• During any unforeseen events (such as floods earthquakes, pandemic,
unavailability of contractors, power failure, etc.), appropriate steps will be taken
by the day-to-day manager to manage any risks to products, and to identify any
non-conforming or suspected non-conforming product.
• Where product may be affected, the RMP verifier is notified with an incident
report including:
− a description of the problem and any affected product;
− a summary of the assessment made; and
− any corrective actions taken to prevent the recurrence of the non-
conformance.
Corrective actions
• Corrective actions will be taken to minimise the occurrence of non-conformance.
• The corrective actions may include:
− amending procedures to correct deficiencies;
− increasing the frequency of inspections or internal audits;
− revising supervision or training programmes when staff, visitors or
contractors are not following GOP as required;
− managing repeat non-conformances; and
− a series of escalating responses for repeated non-conformances.
Determining if a recall is required
• A recall is considered when the Day-to-day Manager believes that products have
been released that have a food safety problem or are not fit for their intended
purpose. Examples of food safety problems include:
− a breach of a regulatory limit; presence of foreign matter that could cause
harm;
− levels of a chemical that could cause harm;
− presence of a microorganism that could make someone sick etc.
• A risk assessment is done to determine if a recall is needed:
− information is gathered to assist in understanding the source and extent of
the problem;
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 51
− refer to MPI Recall Guidance Material
(www.mpi.govt.nz/food-safety/food-recalls/);
− the RMP verifier is contacted for assistance.
• Identification of affected product will be started. Any stock still
on hand will be held until a decision has been made on whether to recall product.
Recall
• If it is determined that a recall of carcass is likely, the Day-to-day Manager is
responsible for working with the operator and the next recipient in the supply
chain to identify and locate animal product.
• Consider how reprocessing or disposal of the animal product will the
management.
• You will need to notify an Animal Products Officer or the MPI Director General as
soon as possible and within 24 hours of the situation being discovered. Contact
New Zealand Food Safety on 0800 00 83 33 or at Food.Recalls@mpi.govt.nz.
Simulated Recall
• A simulated, mock, or trial recall is done at least every 12 months to demonstrate
the effectiveness of the traceability and recall process.
• Refer to MPI Simulated Food Recall Guidance
(www.mpi.govt.nz/food-business/food-recalls/doing-food-recall/)
• Effectiveness is measured by:
− the time taken to trace affected product;
− the time taken to complete the mock recall of affected product; and
− the proportion of product that would have been successfully recalled.
Who’s responsible?
Record the name or position of the person(s) responsible for co-ordinating recalls
_________________________________________________________________
_________________________________________________________________
Monitoring for Operator Verification
• Compliance with these procedures is checked at least ______________ by the
responsible person.
Things to show your verifier
• Load-out dockets or consignment notes for carcasses.
• Diary detailing all communication about the recall and copies of all written
correspondence.
• Recall review notes.
• Inventory records.
• Records of assessment and disposition of non-conforming products.
• Records of recall activities, including mock recall.
• Any correspondence with the RMP verifier or MPI.
Risk Management Programme for Micro Abattoirs – Supporting Systems Page 52
N. Corrective Action
Useful things to know
• To ensure that if problems occur, they are managed appropriately (e.g.
restoration of control, product disposition and prevention of recurrence).
Rules you must follow
Corrective action
• Problems are normally identified by persons as they carry out, monitor or verify
the effectiveness of the tasks documented in the RMP. They may also be
detected through customer complaints.
• When problems occur, corrective actions are carried out in an effective and
timely manner.
• Details of corrective actions are recorded (e.g. in a register). This includes any
follow-up checks used to make sure the corrective actions are working (e.g.
internal audits, external audits).
• Problems detected through the normal day-to-day operation of the RMP are
addressed by a suitably skilled person who will:
− assess the problem;
− restore control;
− identify and retain any suspect product, and determine the product
disposition appropriate to the nature of the problem and the intended use of
the product (e.g. reject, or release as is);
− take action to stop the problem from recurring (e.g. increase surveillance of
the system, make changes to the system, etc.); and
− record the corrective actions (including restoration of control, product
disposition and prevention of recurrence) in the e.g. Corrective Action
Register.
Corrective action for unforeseen circumstances
• The RMP is not written to cover unusual events such as floods, fires or
earthquakes. If such an event happens, appropriate corrective actions are
determined on a case-by-case basis and taken.
• When problems occur due to unforeseen circumstances, the Day-to-day Manager
nominates a suitably skilled person to carry out the “normal” corrective actions
(see above) and to be responsible for:
− completing an in-depth assessment of the suspect product by reviewing
relevant processing records, analyses undertaken, inspecting the product,
advice from experts, literature review etc.;
− ensuring product disposition appropriate to the nature of the problem and
the intended use of the product (e.g. rework, reject, release under restricted
conditions, regrade for alternative use where permitted under the RMP,
etc.); and
− reporting the following to the RMP verifier:
• a description of the problem and the affected product;

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 53
• a summary of the assessment made;
• the decision on the disposition of the product; and
• any actions taken to prevent recurrence of the non-compliance.
Who’s responsible?
Record the name or position of the person(s) responsible for completing Corrective
Action reports ________________________________________________________
_________________________________________________________________
_________________________________________________________________
Things to show your verifier
• Any problems detected and any corrective action taken.
• Any reports given to the RMP verifier.
Examples of these forms can be found in the RMP Operator Resource Toolkit.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 54
O. Storage
Useful things to know
• To ensure the storage environment will maintain the intended state of
preservation and prevent contamination so that products and materials remain
fit for purpose.
Rules you must follow
General requirements
• People hygienically handle product.
• People with any condition or illness of public health concern do not handle any
unprotected product. Refer to B. Personnel Health and Hygiene.
Storage and handling
• All products and materials remain identifiable at all times.
• Products and materials are stored in a manner that:
− minimises contamination and deterioration (e.g. by separation);
− minimises damage to packaging;
− facilitates effective cleaning; and
− facilitates effective inventory control.
• Chemicals and maintenance compounds are stored in a way that minimises
contamination.
Refrigerated or ambient storage
• Any chilling of product is conducted without unnecessary delay and in a manner
that minimises deterioration.
• Any defined temperature is reached as quickly as necessary to ensure the
product remains fit for purpose and does not deteriorate.
Storage of waste materials
• All waste materials are covered in a pest-
proof containers, regularly collected and
disposed of. Refer to G. Cleaning and Sanitation.
Controlling non-conforming product
• Refer to M. Non-conforming Product and Recall.
Monitoring for Operator Verification
• Compliance with these procedures is checked at least ______________ by the
responsible person.

Risk Management Programme for Micro Abattoirs – Supporting Systems Page 55
Things to show your verifier
• Inventory records.
• Completed e.g. Vermin Control Register.
• Completed e.g. Cleaning and Maintenance Records.
• Any problems detected and corrective action taken. Refer to N. Corrective
Action.
Examples of these forms can be found in the RMP Operator Resource Toolkit.
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 56
Part 3: Regulatory Limits and Hazard
Analysis
1. Additional Scope of the RMP
Intended Consumer
Intended consumer
• Humans (general public)
Intended Use
Intended use of product
that leaves RMP
• Ingredient for preparation of other foods (cooked)
Regulatory Limits
Regulatory limits
• None
Other regulatory
requirements specific to
product
• Every consignment must comply with the Animal Products
Notice: Production, Supply and Processing
Labelling requirements
• Labelling of transportation outers as per the Animal
Products Notice: Production, Supply and Processing
Market Eligibility
• New Zealand and/or export to countries that do not require
Official Assurances.

Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 57
Processes and Activities
The RMP covers the following processes and activities for Micro Abattoirs
(tick all applicable processes or activities)
☐
Receiving animals
☐
Holding in pens
☐
Ante-mortem inspection
☐
Slaughter
☐
Killing in the field (mobile premises only)
☐
Dressing
☐
Post-mortem inspection
☐
Packing/labelling
☐
Cooling
☐
Storage
☐
Dispatch
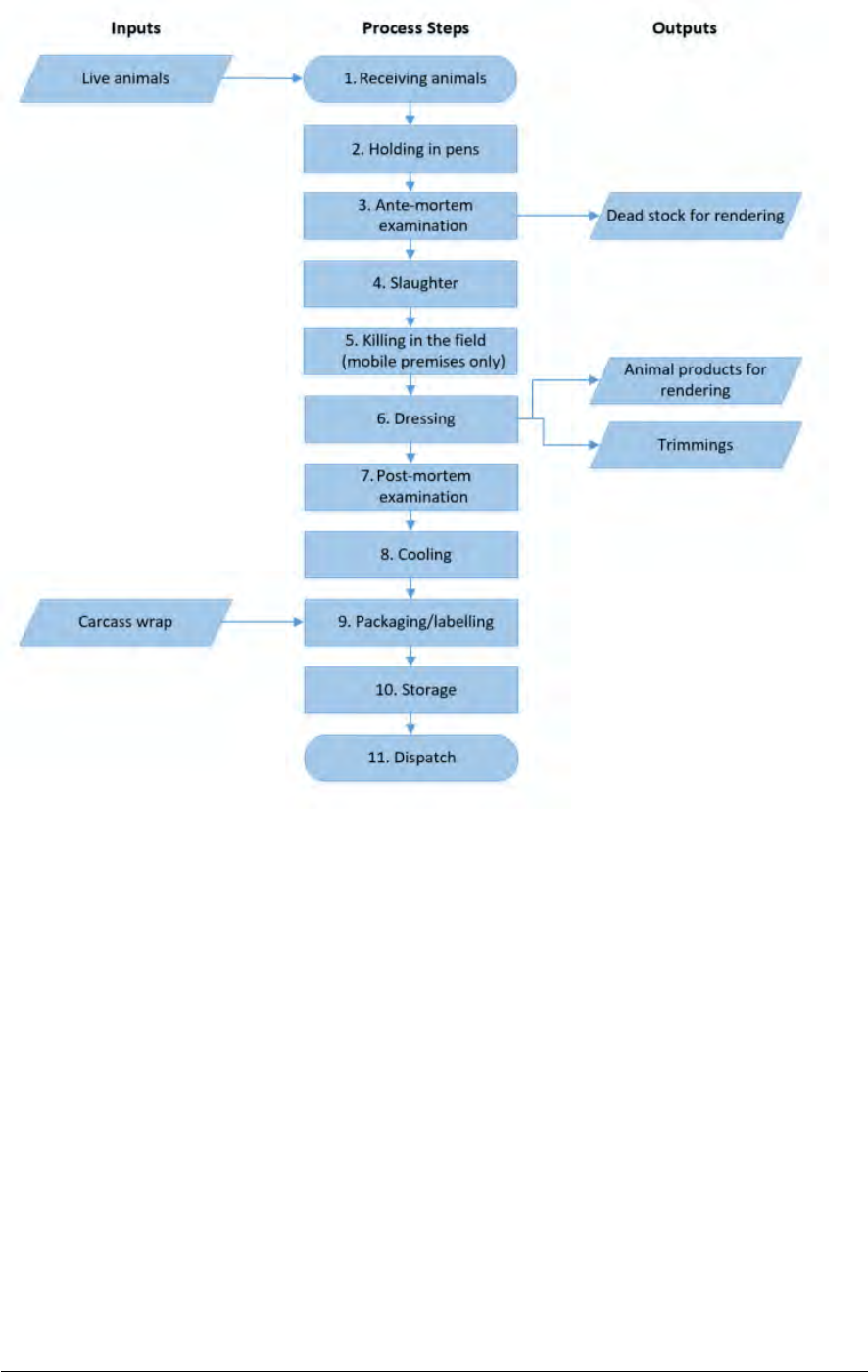
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 58
Generic Process Flow Diagram:
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 59
2. Process Control
Useful things to know
• To ensure animals are slaughtered in accordance with the Code of
Welfare Commercial Slaughter 2018
(www.mpi.govt.nz/dmsdocument/46018).
• To ensure ante-mortem and post-mortem examinations are
undertaken on all animals.
• To ensure traceability of animal material and animal products throughout the
process.
• To ensure that appropriate controls are included in the RMP so that products are
fit for their intended purpose.
Rules you must follow
1. Receiving animals
• Refer to H. Receipt of incoming materials and live animals.
3. Ante-mortem examination
• All animals undergo ante-mortem examination by a qualified ante-mortem
examiner prior to slaughter to assess their suitability for slaughter.
• All ante-mortem and post-mortem examiners have the freedom, access and
authority to carry out their responsibilities as required by the
Animal Products Notice: Production, Supply and Processing
(https://www.mpi.govt.nz/dmsdocument/50182).
• The ante-mortem examination is carried out within 24 hours of the
arrival of an animal at the slaughter premises and within 24 hours
before the slaughter of the animal.
• The ante-mortem examiner assesses each animal for any abnormality that may:
− constitute a food safety hazard in any resulting animal material or animal
product;
− contaminate any animal material or animal product through the dressing of
the animal;
− affect the processing environment to the extent that it may create a hazard
in any animal material or animal product; or
− be detrimental to the welfare of the animal.
• On completion of the ante-mortem examination (or re-examination) of an
animal, and taking into account any information supplied in the relevant ASD, the
ante-mortem examiner makes a decision regarding the suitability for processing
of the animal:
− is suitable for slaughter for human consumption;
− is suitable for slaughter pending treatment for, or recovery from, an
abnormal condition, and, if appropriate, specifies when the animal must be
submitted for re-examination;
− must be slaughtered without delay to prevent the deterioration of an
abnormal condition provided:

Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 60
• the condition does not prevent all or part of the carcass being fit for
human;
• consumption; and
• processing of the carcass will not detrimentally affect the hygiene of
the processing environment.
− is suspect animal material, and is required to be slaughtered at a time
designated by the ante-mortem examiner; or
− is not fit for slaughter for human consumption and should be disposed of in
an appropriate manner.
• The ante-mortem examiner:
− determines the appropriate manner of disposal of the animal that is not
suitable for human consumption;
− records the disease and defect information and provides this information to
MPI in the format required by MPI for that purpose; and
− provides sufficient information to the post-mortem examiner, prior to post-
mortem examination, about the status of the animal, including whether the
animal is:
• a suspect animal (together with the reasons for being suspect);
• a Tb reactor;
• vaccinated for Johne’s disease;
• on a chemical residue list;
• on a disease surveillance suspect list; or
• subject to any other restrictions or conditions described on the
Animal Status Declaration (ASD) form.
• The RMP Manager ensures that animals condemned in the yards during ante-
mortem examination are tagged in a manner that allows clear identification of its
status (i.e. condemned) and facilitates traceability.
• Injured, diseased, moribund and dead animals are handled and disposed of, in
accordance with the procedures summarised in Table 3.1 (HC = human
consumption).
Table 3.1: Handling and disposition of injured, diseased, moribund and dead
animals
Scenario A
Condition of animal
• An animal is injured while in the care of the
operator, or has suffered injury during
transportation to the slaughter premises, and is
deemed suitable for slaughter for human
consumption.
Action Required
• Animal is slaughtered without delay.
Disposition of animal
material
• Animal can be processed for human
consumption.
Scenario B
Condition of animal
• An animal develops a metabolic disorder while in
the care of the operator, or has suffered a

Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 61
metabolic disorder during transport to the
slaughter premises, and is deemed suitable for
slaughter for human consumption.
Action Required
• Animal is slaughtered without delay.
Disposition of animal
material
• Animal can be processed for human
consumption.
Scenario C
Condition of animal
• An animal develops a metabolic disorder while in
the care of the operator, or has suffered a
metabolic disorder during transport to the
slaughter premises, and is deemed suitable for
slaughter pending treatment for, or recovery
from, the disorder.
Action Required
• Animal can be treated and then submitted for
re-examination after a period advised by the
ante-mortem examiner. Depending on the
outcome of the re-examination, the action
required for scenario B or D will apply.
Disposition of animal
material
• Disposition for scenario B or D, as applicable.
Scenario D
Condition of animal
• An animal: is dead or dies in the slaughter
premises (i.e. not slaughtered); or becomes
moribund in the slaughter premises; or is injured
or diseased; and deemed not suitable for
slaughter for HC; and it is not possible to return
the animal to its owner or supplier on animal
welfare grounds.
Action Required
• Injured, diseased or moribund animal is
slaughtered without delay.
• The dead or slaughtered animal can be dressed
in the premises (e.g. to recover the hide or pelt)
in accordance with the following:
• the animal is handled and dressed in a manner
and at a time (e.g. end of the day) that prevents
direct or indirect contamination of animal
materials or products for HC and the carcass and
all parts of the animal are clearly identified and
separated from products for HC throughout the
process.

Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 62
Disposition of animal
material
• Animal material is not suitable for HC, and is
disposed of in an appropriate manner (e.g.
dispatched for rendering or burial), as advised by
the ante-mortem examiner.
• No animal is removed from the premises without the approval of the ante-
mortem examiner.
• The following records are kept for at least 4 years:
− ante-mortem examination report for each animal or group of animals;
− disease and defect information, which is provided to MPI;
− records that provide the following information for each mob of animals:
• the current ante-mortem status of the animals;
• name and signature of the ante-mortem examiner and the date of
examination; and
• relevant information that may assist in the final assessment of
suitability for
• processing.
4. Slaughter (applies to animals slaughtered within the slaughter premises)
• An animal is slaughtered (i.e. stunning, sticking and bleeding) without
unnecessary delay from the time of its arrival at the premises or presentation for
slaughter and in a way that minimises the contamination of the carcass.
• Animals are rendered insensitive (i.e. stunned) before bleeding and kept in this
state until death.
• Whenever stunning becomes inadequate, the slaughter of animals is stopped
until the problem is rectified.
• When blood is collected for human consumption, the operator ensures that:
− blood is not collected from animals condemned for disease conditions or
from a reactor to a diagnostic test;
− blood does not come into contact with the outer surface of any slaughtered
animal or become contaminated in any way;
− traceability between the blood collected and source animal(s) is maintained
until the animal(s) has passed post-mortem examination;
− when batch collection is undertaken, all source animals contributing to the
batch meet requirements (a)-(c), otherwise the entire batch is condemned;
− equipment used for the collection of blood is disinfected after each batch;
and
− any equipment, such as a knife, that comes into direct contact with exposed
parts of the animal, is cleaned and sanitised before the next animal is bled.
• Animals that are identified by the ante-mortem examiner as suspect:
− are processed last (i.e. at the end of the processing day);
− are handled in a way that prevents cross-contamination between suspect
animals and non-suspect animals (and their parts), and between different
suspect animals; and
− have their carcasses and parts identified as suspect materials

Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 63
5. Killing in the field
• After ante-mortem examination, an animal may be killed on an adjacent field or
paddock by the mobile slaughter operator using a gun in
accordance with the requirements of the Code of Welfare
Commercial Slaughter 2018
(www.mpi.govt.nz/dmsdocument/4601)
• Use solid bullets (not frangible) where an animal is killed by
shooting with a gun. This minimises the potential for contamination of the
product with bullet fragments.
• Killing, sticking and bleeding are done in a manner that minimises the
contamination of the carcass (e.g. contamination through the stick wound).
• Blood for human consumption is not collected from an animal killed on the field.
• Opening cuts on the animal (except for the opening cut for sticking) are not
allowed to be made while on the field.
• Transfer or convey the killed animal to the mobile slaughter facility in a manner
that minimises the contamination and deterioration of the carcass (e.g. it is not
dragged through mud).
6. Dressing
• Carry out dressing of carcasses:
− without unnecessary delay and in a hygienic manner to minimise the
transfer, proliferation and redistribution of contaminants on and between
animal material or product;
− in accordance with the principles and guidance provided in the
Red Meat Code of Practice Chapter 5: Slaughter and Dressing
(www.mpi.govt.nz/dmsdocument/21659); and
− in compliance with the general and species, specific
requirements for presentation for post-mortem examination
given in the Red Meat Code of Practice Chapter 6: Presentation
for Post-Mortem Examination
(www.mpi.govt.nz/dmsdocument/7491)
• Maintain traceability for all parts of an animal (or animals in case of batch
processing) presented for post-mortem examination (i.e. animal parts are
positively identifiable to the carcass or to the group of animals as appropriate)
until post-mortem examination is complete.
• Apply hygienic techniques during dressing to minimise contamination of the
carcass from:
− contaminated parts of the animals, such as the hide, pelt or hair, the gastro-
intestinal tract, the integument, hooves, trotters, or feet of the same or
another carcass;
− contaminated equipment, such as uncleaned knives, viscera tables, buggies
and equipment used for suspending carcasses, offal or other parts;
− contaminated surfaces, such as the floor or drains; and
− wastes and other contaminated material.
• Perform the operations posing the least risk of contamination first where
multiple operations are carried out on the same carcass by the same person.

Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 64
• Collect offal and other animal materials for human consumption in a hygienic
manner.
• Physically separate carcasses and animal products that have not passed post-
mortem examination from those that have passed post-mortem examination.
• Collect scraps, trimmings and other animal materials that are not suitable for
human consumption in designated containers and dispose of them appropriately.
7. Post-mortem examination
• Post-mortem examination is performed on all carcasses and their parts by a
qualified post-mortem examiner.
• Prior to undertaking any post-mortem examination, the post-mortem examiner
considers any relevant information provided by the ante-mortem examiner.
Refer to the Ante-mortem Examination supporting system above.
• Post-mortem examination is undertaken:
− in a way that minimises cross-contamination between carcasses; and
− in accordance with the procedures given in the Red Meat Code
of Practice Chapter 7: Post-mortem Examination
(www.mpi.govt.nz/dmsdocument/7494); and
− without delay following the dressing of an animal except when
the bullet point immediately below applies;
• Post-mortem examination of a carcass or group of carcasses and their parts may
be delayed till the end of the processing day (the same day they are killed)
provided that all the animal material are held:
− with adequate separation and identification (i.e. all parts remain positively
identifiable to the carcass until completion of post-mortem examination);
− under hygienic conditions to prevent cross-contamination; and
− at a temperature of 7°C or cooler to prevent microbiological growth on the
product and product deterioration.
• Post-mortem disposition of animal material and products is made in accordance
with the Red Meat Code of Practice Chapter 8: Post-Mortem
Dispositions (www.mpi.govt.nz/dmsdocument/7497).
• The RMP Manager ensures that retained products pending the
decision on disposition are tagged in a manner that allows clear
identification of its status (i.e. retained) and facilitates traceability.
• All condemned viscera, carcasses and parts of carcasses are clearly identified by
applying approved branded ink (e.g. by use of a “Condemned” stamp.)
• All products remain under the control of the post-mortem examiner until the
assessment is completed by the examiner and a decision is made regarding their
fitness for human consumption.
• Post-mortem records of defects or diseases found during inspection are kept for
at least 4 years.
8. Cooling of carcasses and offal
• Carcasses and offal are cooled in a refrigeration unit where the air
temperature is 7°C or cooler.
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 65
• Carcasses are cooled to a deep meat temperature (DMT) of 7°C from the time
post-mortem examination is completed, within the time period specified in the
table below. The DMT of a carcass is measured at the thermal centre of the
largest muscular
mass.
• There are procedures for achieving the above cooling rates. These procedures
include the different parameters, such as the air temperature, air speed and
movement, loading configuration (e.g. number of carcasses, spacing), and
packaging of products.
Table 3.2: Time for carcass to reach a DMT of 7°C
Carcass size Time to reach a DMT of 7°C
Small carcasses (e.g. sheep, goats,
bobby calves, small deer, pigs
other than choppers, emu,
ostriches, alpaca)
24 hours
Large carcasses (e.g. cattle,
buffalo, horses, large deer such as
Wapiti deer, large pigs such as
choppers)
48 hours
Table 3.3: DMT at loadout
Product type DMT at loadout
Chilled mammal, ostriches, emus
and poultry
7°C or cooler
Frozen mammals, ostriches, emus
and poultry
-12°C or cooler
Table 3.4: Scenarios for releasing carcasses that have not reached the required
preservation temperature
Scenario Requirements
Fixed premises
Carcass is partially cooled/chilled
prior to release from the slaughter
premises and then picked up and
transported by another operator
(e.g. a transport operator or a
butcher).
The slaughter operator confirms and
records that the receiving operator (e.g. a
transport operator or a butcher) has a
registered RMP or FCP that covers the
transfer of the product, or operates under
the
relevant National Programme under the
Food Act 2014.
Mobile premises
Carcass is released from the
slaughter premises directly to the
The slaughter operator confirms and
records that the meat is solely for the
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 66
owner of the animal (e.g. the
farmer) immediately after slaughter
and dressing with minimal or no
chilling, and the owner intends
to
use the product only for his/her
personal use.
animal owner’s personal
use and will not be traded.
Carcass is released from the
slaughter premises directly to the
owner of the animal (e.g. the
farmer) immediately after slaughter
and dressing with minimal or no
chilling, and the owner intends to
trade or use the product for
commercial purposes.
The slaughter operator confirms and
records that the animal owner has a
registered RMP or FCP that
covers further processing of the product, or
operates under a relevant National
Programme under the Food Act 2014.
Carcass is partially cooled/chilled
prior to release from the slaughter
premises to another operator (e.g. a
butcher).
The slaughter operator confirms and
records that the receiving operator has a
registered RMP or FCP that covers further
processing of the product, or operates
under a relevant National Programme
under the Food Act 2014.
9. Packing and labelling
• Where carcasses are wrapped:
− only new materials suitable for food contact use are used for wrapping;
and
− condensation or frosting on carcasses is controlled, particularly when
non-permeable materials (e.g. polythene wrap) are used.
• Containers, such as plastics bins, pails and plastic bags used for packing offal,
blood and other products are:
− suitable for food contact use;
− clean;
− leak-proof; and
− provided with a cover, when necessary to protect the product from
contamination during storage and transport.
• Transportation outers, when used, are labelled with the following information:
− the product name or description;
− storage directions; and
− the slaughter date or other form of batch/lot identification.
• Carcasses and other products that cannot be practically labelled, have the
information above provided on tickets attached to the carcass, as well as on
accompanying documentation.
• The labels of transportation outers and accompanying documentation for
products that are not intended for human consumption (e.g. for petfood use)
clearly state “Not for human consumption”.

Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 67
10. Storage
• All products are handled and stored in the chiller or freezer
in a way that
minimises their contamination or deterioration.
• Products are:
− moved to the chiller or freezer as soon as possible after post-mortem
examination or packing, as applicable;
− held at appropriate temperatures to maintain their safety and suitability for
their intended purpose;
− protected against contamination or damage;
− stored in a way that ensures that exposed products have no contact with the
floor, walls, and contaminated
surfaces;
− kept separate from maintenance compounds and other hazardous materials;
and
− properly labelled or identified.
• The operator checks that the following conditions are met prior to release of
products from the slaughter premises:
− Products are properly packed and labelled;
− Product loadout temperature is taken and ed (e.g. DMT of carcasses);
− The transport vehicle is clean and does not contain any material that could
contaminate and of the product and affect its suitability for human or animal
consumption; and
− Products are accompanied by relevant documents that ensure traceability.
Things to show your verifier
• Qualifications from ante-mortem examiner
• Demonstrate how you safely slaughter an animal.
• Demonstrate how you safely kill an animal in the field.
• Any records or training that has been completed (e.g. post-mortem)
• Traceability records and traceability processes you follow
• Demonstrate how you check the temperature of the carcasses during
refrigeration and cooling.
11. Dispatch
• If the suitability or intended purpose of a product changes from its original
intended purpose (e.g. human consumption product is downgraded to petfood
use), any
labelling or other identification that indicates the product as suitable
for human consumption is removed or defaced and the product’s new status is
indicated in all labelling and accompanying documentation. This is carried out at
the earliest opportunity, and prior to the release of the product from the
premises.

Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 68
3. Risk Factor Identification and Control
Useful things to know
• To identify the risk factors (from hazards to human and animal health,
wholesomeness, false and misleading labelling) that are reasonably likely to
occur at each process step (including all inputs).
Rules you must follow
Risks from hazards to human and animal health
• A hazard analysis and critical control point (CCP) determination has been
conducted (see Table 3.5). No CCPs were identified.
• Good operating practices (GOP) are followed as outlined in the RMP Part 2:
Supporting Systems
• All identified hazards are expected to be adequately controlled by the control
measures listed in Tables 3.5.
Risks to wholesomeness
• Risk factors have been identified (see Table 3.6)
• All identified risk factors are expected to be adequately controlled by following
good operating practices (GOP) as outlined in the RMP Part 2: Supporting
Systems and the control measures listed in Table 3.6.
Risks from false and misleading labelling
• Risk factors have been identified (see Table 3.7)
• All identified risk factors are expected to be adequately controlled by following
good operating practices (GOP) as outlined in the RMP Part 2: Supporting
Systems and the control measures listed in Table 3.7.
Things to show your verifier
• Completed records of good operating practices.
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 69
Table 3.5: Hazard analysis and CCP determination for Micro Abattoirs
Process step
Inputs
Hazard reasonably
likely to occur on or
in the product at
this step
Justification
Q1. Is there a control
measure(s)
for the
hazard at this step?
If yes, identify the control
measure and then
answer Q2.
If no, consider hazard at
next step.
Q2. Is the control
measure at this step
essential to food
safety as defined by a
regulatory limit?
If yes, this step is a
CCP.
If no, this step is not a
CCP.
CCP
no.
1. Receiving
animals
Live animal,
ante-mortem
passed dead
animal
B – Bacterial
pathogens – grossly
detectable
abnormalities
Bacterial pathogens
associated with faeces,
ingesta and dirt from
gastro intestinal tract
and hide, e.g.
Salmonella spp.,
Campylobacter jejuni, E.
coli O157:H7
Bacterial pathogens
associated with grossly-
detectable
abnormalities (fever,
abscesses, navel
infections), e.g.
Salmonella spp. for
fever
No
No
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 70
1
Currently, potential hazards associated with bacteraemia cannot be adequately addressed by any control measure applied during the slaughter and dressing process
including post-mortem examination. Therefore, this hazard will not be considered further in this generic RMP.
2
Beef carcasses are inspected for Taenia saginata during post-mortem, but existing inspection methods have low sensitivity to low grade infection of cattle. In certain
circumstances, T. saginata may still be present in the inspected and passed carcass. In these cases, a HACCP-based programme for further detection and removal of T.
saginata may be applicable during boning. However, for the purposes of this generic model, and considering the rare occurrence of this hazard in beef, this hazard will
not be considered any further in the hazard analysis.
3
Toxoplasma gondii in deer or sheep cannot be adequately addressed by any control measure applied during the slaughter and dressing process, including post-mortem
examination. However, freezing to ≤ -12°C will render tissue cysts of T. gondii nonviable. To avoid repetition in the table, the hazard is not carried through each step.
Table 3.5: Hazard analysis and CCP determination for Micro Abattoirs
Process step
Inputs
Hazard reasonably
likely to occur on or
in the product at
this step
Justification
Q1. Is there a control
measure(s)
for the
hazard at this step?
If yes, identify the control
measure and then
answer Q2.
If no, consider hazard at
next step.
Q2. Is the control
measure at this step
essential to food
safety as defined by a
regulatory limit?
If yes, this step is a
CCP.
If no, this step is not a
CCP.
CCP
no.
Bacterial pathogens
associated with
bacteraemia
1
, e.g.
Salmonella spp
B – Pathogenic
parasites
e.g. Taenia saginata
Toxoplasma gondii
No
2,3
No

Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 71
4
The control of chemical residues involves effective farming practices and the monitoring of chemical residues under the National Residue Monitoring and Surveillance
Programme. Sporadic chemical residues at some level will always occur, but results from the programme indicate that residue levels in farmed mammals are generally in
compliance with national requirements. Therefore, they have not been considered further at subsequent steps in this RMP template.
Table 3.5: Hazard analysis and CCP determination for Micro Abattoirs
Process step
Inputs
Hazard reasonably
likely to occur on or
in the product at
this step
Justification
Q1. Is there a control
measure(s)
for the
hazard at this step?
If yes, identify the control
measure and then
answer Q2.
If no, consider hazard at
next step.
Q2. Is the control
measure at this step
essential to food
safety as defined by a
regulatory limit?
If yes, this step is a
CCP.
If no, this step is not a
CCP.
CCP
no.
C – Chemical
residues
Antibacterial products
(e.g. sulphonamide)
Controlled under the
national residue
program
4
.
Supplier declarations
No
2. Holding in
pens
Live animal
B – Bacterial
pathogens – grossly
detectable
abnormalities
Micro carried over from
previous step.
No
No

Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 72
5
Grossly detectable abnormalities are addressed during ante-mortem and post-mortem examinations, which are currently the responsibility of the regulator. Therefore,
they will only be considered at the ante- and post-mortem steps in this RMP template. However, if ante-mortem and post-mortem examinations are undertaken by the
company (i.e. operator’s responsibility), then these steps must be considered during hazard analysis.
Table 3.5: Hazard analysis and CCP determination for Micro Abattoirs
Process step
Inputs
Hazard reasonably
likely to occur on or
in the product at
this step
Justification
Q1. Is there a control
measure(s)
for the
hazard at this step?
If yes, identify the control
measure and then
answer Q2.
If no, consider hazard at
next step.
Q2. Is the control
measure at this step
essential to food
safety as defined by a
regulatory limit?
If yes, this step is a
CCP.
If no, this step is not a
CCP.
CCP
no.
3. Ante-
mortem
examination
Live animal
B – Bacterial
pathogens – grossly
detectable
abnormalities
Micro carried over from
previous step.
Controlled under the
antemortem examination
system
5
.
No
4. Slaughter
Live animal
B – Bacterial
pathogens
Micro contamination of
the carcass from the
fleece/pelt is likely to
occur during sticking.
Yes – correct sticking
technique will minimise
contamination.
No
5. Killing in the
field
Live animal
B – Bacterial
pathogens
Micro contamination of
the carcass from the
fleece/pelt is likely to
occur during sticking.
Yes – correct sticking
technique will minimise
contamination.
No
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 73
Table 3.5: Hazard analysis and CCP determination for Micro Abattoirs
Process step
Inputs
Hazard reasonably
likely to occur on or
in the product at
this step
Justification
Q1. Is there a control
measure(s)
for the
hazard at this step?
If yes, identify the control
measure and then
answer Q2.
If no, consider hazard at
next step.
Q2. Is the control
measure at this step
essential to food
safety as defined by a
regulatory limit?
If yes, this step is a
CCP.
If no, this step is not a
CCP.
CCP
no.
Micro contamination of
the carcass from the
field environment.
Careful handling of the
carcass.
6.a Dressing
Carcass /
head / offal
B – Bacterial
pathogens
Micro contamination of
the carcass from the
fleece/pelt is likely to
occur when making the
opening cuts and during
flaying.
Yes – correct flaying
techniques and
prevention of rollback
will minimise
contamination.
No
Carcass /
head / offal
B – Bacterial
pathogens
Micro contamination of
the carcass from the
fleece/pelt is likely to
occur at this step.
Yes – correct rip down
techniques and
prevention of rollback
will minimise
contamination.
Yes – correct pelting
techniques will minimise
contamination.
No
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 74
Table 3.5: Hazard analysis and CCP determination for Micro Abattoirs
Process step
Inputs
Hazard reasonably
likely to occur on or
in the product at
this step
Justification
Q1. Is there a control
measure(s)
for the
hazard at this step?
If yes, identify the control
measure and then
answer Q2.
If no, consider hazard at
next step.
Q2. Is the control
measure at this step
essential to food
safety as defined by a
regulatory limit?
If yes, this step is a
CCP.
If no, this step is not a
CCP.
CCP
no.
Carcass /
head / offal
B – Bacterial
pathogens
Micro carried over from
the previous step.
Yes – hygienic trimming
will remove any visible
faecal contamination and
reduce micro
contamination on the
carcass.
No
6.b Dressing
(Evisceration)
Carcass /
offal
B – Bacterial
pathogens
Micro contamination
from the GIT can occur
at this step.
Yes – hygienic techniques
during freeing and
dropping of the bung and
prevention of puncturing
the GIT will minimise
contamination.
No
7. Post-
mortem
examination
Carcass
B – Bacterial
pathogens - grossly
detectable
abnormalities
Micro carried over from
the evisceration step.
Controlled under the
post-mortem
examination system.
No
B – Bacterial
pathogens
Micro carried over from
the previous step.
Yes – hygienic trimming
will remove any visible
No
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 75
Table 3.5: Hazard analysis and CCP determination for Micro Abattoirs
Process step
Inputs
Hazard reasonably
likely to occur on or
in the product at
this step
Justification
Q1. Is there a control
measure(s)
for the
hazard at this step?
If yes, identify the control
measure and then
answer Q2.
If no, consider hazard at
next step.
Q2. Is the control
measure at this step
essential to food
safety as defined by a
regulatory limit?
If yes, this step is a
CCP.
If no, this step is not a
CCP.
CCP
no.
faecal contamination and
reduce micro
contamination on
affected parts of the
carcass.
8. Cooling
Carcass
Red offal
B – Bacterial
pathogens
Micro carried over from
the previous step.
Growth of mesophiles
can occur if there is
cooling failure.
Yes – effective cooling
will prevent the growth
of mesophiles.
No
9. Packing
Carcass
Red offal
B – Bacterial
pathogens
Micro carried over from
the previous step.
No
No
10. Storage
Carcass
Red offal
B – Bacterial
pathogens
Micro carried over from
the previous step.
Micro growth can occur
if there is refrigeration
failure.
Yes – effective
refrigeration will prevent
micro growth
No
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 76
Table 3.5: Hazard analysis and CCP determination for Micro Abattoirs
Process step
Inputs
Hazard reasonably
likely to occur on or
in the product at
this step
Justification
Q1. Is there a control
measure(s)
for the
hazard at this step?
If yes, identify the control
measure and then
answer Q2.
If no, consider hazard at
next step.
Q2. Is the control
measure at this step
essential to food
safety as defined by a
regulatory limit?
If yes, this step is a
CCP.
If no, this step is not a
CCP.
CCP
no.
B –
Toxoplasma gondii
in chilled products
Hazard carried over
from previous step.
No
No
11. Dispatch
Carcass
Red offal
B – Bacterial
Pathogens
Micro carried over from
the previous step.
Micro growth can occur
if temp abuse occurs.
Yes – time/temperature
control during loadout
will prevent micro
growth.
No
B –
Toxoplasma gondii
in chilled products
Hazard carried over
from previous step.
No
No
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 77
Table 3.6: Summary of identified risk factor and controls related to wholesomeness
Risk factor
Source or cause of risk factor
Control measures for preventing/ minimising the
risk factor
Spoilage of carcass or offal
Micro contamination of
product during dressing and
subsequent handling.
GOP – hygienic dressing, cutting, boning, and
handling.
See Part 3 – 5. Dressing
Micro growth due to improper
time/temperature control.
GOP – time/temperature control, proper
refrigeration.
See Part 3 – 9. Cooling of Carcasses and Offal.
See Supporting System O. Storage
Wholesomeness defects (e.g. blood clots,
bruises, hair).
Improper handling of live animals and
dressing of carcasses.
GOP – handling of stock, hygienic dressing,
trimming.
See Part 3 – 5. Dressing
See Supporting System H. Receipt of Incoming
Materials and Live Animals.
Risk Management Programme for Micro Abattoirs – Regulatory Limits and Hazard Analysis Page 78
Table 3.7: Summary of identified risk factor and controls from false or misleading labelling
Risk factor
Source or cause of risk
factor
Control measures for preventing/ minimising the risk factor
Incorrect details on label or
transportation outers, for
example:
• type of product
• claims (e.g. organic)
• product description
• lot identification or batch
number
• storage directions
Incorrect label design
Procedures for ensuring correct label design and compliance with regulatory
requirements
Product labelled with
wrong ticket
Procedures for ensuring correct packaging and labelling of products.
See H. Traceability, Inventory and Labelling.
