
Lecture note- 4
Organic Chemistry CHE 502
CARBOHYDRATES
Co-Coordinator – Dr. Shalini Singh
DEPARTMENT OF CHEMISTRY
UTTARAKHAND OPEN UNIVERSITY

UNIT- 7 CARBOHYDRATES-I
CONTENTS
7.1. Objectives
7.2. Introduction
7.3. Classification and Nomenclature
7.4. Monosaccharides
7.5. Mechanism of osazone formation
7.6. Interconversion of glucose and fructose
7.7. Chain lengthening and chain shortening of glucose
7.8 Summary
7.9. Model examination questions
7.1 OBJECTIVES
After going through this unit you will be able to:
• Define carbohydrates,
• Differentiate and classify the three major groups of carbohydrates,
• Define anomers, mutarotatation, configuration and mechanism of osazone formation,
• Describe ether and ester formation,
• Differentiate between reducing and non reducing sugars,
• Define interconversion of glucose and fructose,
• Describe the chain lengthening and chain shortening of aldose
• Discuss about Erythreo and threo distereomers conversion of glucose
• Determination of ring size of monosaccharides,
7.2 INTRODUCTION
Carbohydrates are a class of naturally occurring organic compounds of carbon, hydrogen
and oxygen which are primarily produced by plants. They are extremely widespread in plants
comprising upto 80% of dry weight. These are ultimate source of our food. In higher animals the
simple sugar glucose is an essential constituent of blood and occurs in a polymeric form as
glycogen in the liver and muscle.
In the green plants, carbohydrates are produced by a process called photosynthesis. This
process involves the conversion of simple compounds CO
2
and H
2
O into glucose (C
6
H
12
O
6
) and
is catalysed by green colouring pigment chlorophyll present in the leaves of plants. The energy
required for this conversion is supplied by sun in the form of sunlight.
Carbohydrates are very useful for human beings. They provide us all the three basic
necessities of life i.e., foog (starch containing grain), clothes (cellulose in the form of cotton ,
linen and rayon) and shelter ( cellulose in the form of wood used for making our houses and
furniture etc.). Carbohydrates are also important to the economy of many nations. For example,
sugar is one of the most important commercial commotidies.
The term carbohydrates arose because the general formula for most of them could be
written as C
x
(H
2
O)
y
and thus they may be regarded as hydrates of carbon. However, this
definition was not found to be correct e.g., rhamnose, a carbohydrate, is having the formula
C
6
H
12
O
5
while acetic acid
having formula
C
2
H
4
O
2
is not a carbohydrate. Simple carbohydrates
are also known as sugars or saccharides (Latin: Saccharum; Greek : Sakcharon, Sugar) and the
ending of the names of most sungars is –ose. Examples: glucose, fructose, sucrose, maltose,
arabinose, etc.
Chemically, carbohydrates contain mainly two functional groups, carbonyl group (
aldehyde or or ketone) and a number of hydroxyl groups. Accordingly carbohydrates are now
defined optically active polyhydroxy aldehydes or polyhydroxy ketones or the compound that
can be hydrolysed to either of them.

7.3 CLASSIFICATION AND NOMENCLATURE
7.3.1 Classification
Carbohydrates, in general, may be classified into two classes:
(i) Sugars. These are crystalline substances which are sweet and water soluble. For
examples, glucose, fructose and cane sugar.
(ii) Non-sugars. These are tasteless, insoluble in water and amorphous. For example. Starch,
cellulose, etc.
However, these days Carbohydrates are systematically classified into three major group:
(a) Monosaccharides. The simplest carbohydrates that cannot be hydrolysed into simpler
carbohydrates, are called monosaccharides.depending upon whether they contain an
aldehyde or keto groups, they may be called aldoses or ketoses. For example, a five
carbon monosaccharide having aldehyde group is called aldopentose and six carbon
monosaccharide containing a keto group is called keto-hexose. A few examples of
monosaccharides are given below:
Aldotetroses. Erythrose and Threose; CH
2
OH(CHOH)
2
CHO.
Ketotetroses. Erythrulose, CH
2
OHCOCHOHCH
2
OH.
Aldopentoses. Ribose, arabinose, Xylose and Lyxose. CH
2
OH(CHOH)
3
CHO.
All have a common molecular formula but different structures.
Ketopentoses. Ribulose and Xylulose; CH
2
OHCO(CHOH)
2
CH
2
OH.
Aldohexoses. Glucose, mannose, galactose; CH
2
OH(CHOH)
4
CHO.
Ketohexoses. Fructose, Sorbose etc. CH
2
OHCO(CHOH)
3
CH
2
OH.
(b) Oligosaccharides. These are the carbohydrates which can be hydrolysed into a definite
number of monosaccharide molecules. Depending upon the number of monosaccharides
that are obtained from them on hydrolysis, they may be called di-, tri- or tetra-
saccharides: For example:

Disaccharides: sucrose, lactose, maltose. All these have the same molecular formula C
12
H
22
O
11
.
Trisaccharides: raffinmose (C
18
H
32
O
16
).
Tetrasaccharides: stachyose (C
24
H
42
O
21
).
(c) Polysaccharides. Carbohydrates that yield a large number of molecules (more than ten
molecules) of monosaccharides on hydrolysis are called polysaccharides. The common
examples are starch, cellulose, glycogen, etc.
7.3.2 Nomenclature
Carbohydrates contain hydroxy and aldehydic or ketonic groups. They are named according to
IUPAC system of nomenclature
Compound
Common name
IUPAC name
CH
2
OHCHOHCHO
Glyceraldehyde
2, 3-dihydroxy propanol
CH
2
OHCOCH
2
OH
Dihydroxyacetone
1,3-dihydroxy propanone
CH
2
OH(CHOH)
4
CHO
Glucose
2,3,4,5,6-pentahydroxyhexanal
CH
2
OH(CHOH)
3
COCH
2
OH
Fructose
1,3,4,5,6-pentahydroxyhexan-2-one
7.4 MONOSACCHARIDES
The monosaccharides are again classified on the basis of two factors:
(1) By the carbonyl function. Those containing the aldehydic function,-CHO, are called
aldoses. Others containing the keto group, -CO-, are called ketoses.
(2) By the number of Carbonyl atoms in the molecule. These monosaccharides containing
3,4,5,6 etc., carbon atoms are designated as trioses, tetroses, pentoses, hexoses, and so on.
Monosaccharides are polyhydric aldehydes and ketones which cannot be hydrolysed into simpler
carbohydrates.
7.4.1 Structures of monosaccharides
The common monosaccharides are given in table.

Table. Monosaccharides
No of
carbon
atoms
Class
Molecular
formula
aldoses
Structural formula
Examples
3
aldotrioses
C
3
H
6
O
3
CH
2
OHCHOHCHO
Glyceraldehyde
4
aldotetroses
C
4
H
8
O
4
CH
2
OH(CHOH)
2
CHO
Erythrose,
Threose
5
Aldopentose
C
5
H
10
O
5
CH
2
OH(CHOH)
3
CHO
Arabinose,
Ribose, Xylose,
Lyxose
6
aldohexoses
C
6
H
12
O
6
CH
2
OH(CHOH)
4
CHO
Glucose,
galactose,
mannose, allose,
talose, gulose,
iodose, etc.
3
ketotrioses
C
3
H
6
O
3
CH
2
OHCOCH
2
OH
dihydroxyacetone
4
ketotetroses
C
4
H
8
O
4
CH
2
OHCOCHOHCH
2
OH
erythrulose
5
ketopentoses
C
5
H
10
O
5
CH
2
OHCO(CHOH)
2
CH
2
OH
Ribulose,
Xylulose
6
ketohexoses
C
6
H
12
O
6
CH
2
OHCO(CHOH)
3
CH
2
OH
Fructose,
Sorbose,
Tagatose, Psicose
7.4.2 Glucose
Glucose is most common monosaccharide. It is known as Dextrose because it occurs in
nature principally as optically dextrorotatory isomer. Glucose is found in most sweet fruits,
especially grapes (20-30%), and honey. It is an essential constituent of human blood. The blood
normally conatains 65 to 110 mg (0.06 to 0.1%) of glucose per 100 ml. In diabetic persons the

level may be much higher. In combined form glucose occurs in abundance in cane sugar and
polysaccharides such as starch and cellulose.
Preparation of Glucose
1. From sucrose (Cane sugar)
When sucrose in boiled with dilute HCl or H2SO4 in alcoholic solution, glucose and fructose are
obtained in equal amounts.
2. From Starch
Glucose is produced commercially by the hydrolysis of starch by boiling it with dilute H2SO4 at
high temperature under pressure.
in this process, an aqueous solution of starch obtained from corn is acidified with dilute H2SO4.
It is then heated with high pressure steam in an autoclave. When the hydrolysis is complete, the
liquid is neutralized with sodium carbonate to pH of 4-5. The resulting solution is concentrated
under reduced pressure to get the crystals of glucose.
Physical properties of glucose
Some important physical properties of glucose are mentioned as under:
1. It is colourless sweet crystalline compound having m.p.419 K.
2. It is readily soluble in water, sparingly soluble in alcohol and insoluble in ether.
3. It forms a monohydrate having m.p. 391 K.
4. It is optically active and its solution is dextrorotatory. The specific rotation of fresh
solution is + 112
0
C.
5. It is about three fourth as sweet as sugarcane i.e., sucrose.
Chemical properties of glucose

Chemical properties of glucose can be studied under the following headings:
(A) Reactions of aldehydic group
1. Oxidation. (a) Glucose gets oxidized to gluconic acid with mild oxiding agents like
bromine water
Only-CHO group is affected.
(b) A strong oxidizing agent like nitric acid oxidizes both the terminal groups viz. –CH2OH and
–CHO groups and saccharic acid or glucaric acid is obtained.
(d) Glucose gets oxidized to gluconic acid with ammonical silver nitrate ( Tollen’s reagent)
and alkaline copper sulphate ( Fehling solution). Tollen’s reagent is reduced to metallic
silver (silver mirror) and Fehling solution to cuprous oxide which is a red precipitate.
(i) With Tollen’s reagent
(ii) With Fehling solution

2. Reduction (a) glucose is reduced to sorbitol or Glucitol on treatment with sodium
amalgam and water.
(b) On reduction with conc. HI and red P at 373 K glucose gives a mixture of n-hexane and 2-
idohexane
3. Reaction with HCN. Like aldehydes, glucose reacts with HCN forming cyanohydrins.
4. Reaction with hydroxylamine. Glucose forms glucose oxime.
(B) Reactions of hydroxyl groups
1. Reaction with acetic anhydride or acetyl chloride. Glucose forms penta acetate with
acetic anhydride of acetyl chloride.
2. Reaction with methyl alcohol. Glucose reacts with methy alcohol in the presence of dry
HCl gas to form methyl glucoside.

3. Reaction with metallic hydroxides. Glucose reacts with calcium hydroxide to form
calcium glucosate which is water soluble.
(C) Miscellaneous reactions
1. Action of acids. On warming with conc.HCl, glucose forms 5-hydroxy methyl furfural,
which on further reaction gives laevulinic acid.
2. Fermentation. Glucose undergoes fermentation into ethyl alcohol in the presence of the
enzyme zymase.
This reaction called alcoholic fermentation is the basis of manufacture of wines and alcohol.
3. Reaction with Alkalies. When warmed with strong sodium hydroxide solution, glucose
forms a brown resinous product. In dilute alkali solution, D-glucose rearranges to give a
mixture of D- glucose, D-mannose and d-fructose.
The above equilibrium is established via the enediol starting from any of these three hexoses.

That is why D-Fructose, although it has a ketonic C=O group, reduces Fehling’s solution or
Tollen’s reagent. The rearrangement reaction of a monosaccharides in weakly alkaline solutions
to give a mixture of isomeric sugars, is named as Lobry de Bruyn Van Ekestein rearrangement.
Structure of glucose
1. On the basis of elemental analysis and molecular weight determination the molecular
formula of glucose is C
6
H
12
O
6
.
2. The reduction of glucose with red phosphorus and HI gives n-hexane.
Therefore, the six carbon atoms of glucose form a straight chain.
3. It forms penta acetate on treatment with acetic anhydride which indicates the presence of
five hydroxyl groups in the molecule.
4. Glucose reacts with hydroxyl amine to form an oxime and with hydrogen cyanide to form
cyanohydrins. It indicates the presence of a carbonyl group. It also forms
phenylhydrazone on treatment with phenylhydrazine.

5. The mild oxidation of glucose with bromine water or sodium hypobromide yields a
monocarboxylic acid (gluconic acid) containing same number of carbon atoms as in
glucose, i.e., six. This indicates that the carbonyl group must be aldehyde group.
6. The catalytic reduction of glucose gives a hexahydric alcohol (sorbitol) which gives
hexaacetate on treatment with acetic anhydride. The sixth hydroxyl group must be
obtained by the reduction of aldehyde group, thus further confirming the presence of an
aldehyde group and five hydroxyl groups in glucose.
7. Oxidation of gluconic acid with nitric acid yields a dicarboxylic acid (glucaric acid) with
the same number of carbon atoms as in glucose. Thus besides aldehyde group, glucose
must contain a primary alcoholic group also, which generates the second carboxylic
group on oxidation.
8. Glucose is a stable compound and does not undergo dehydration easily, indiacating that
not more than one hydroxyl group is bonded to a single carbon atom. Thus all the
hydroxyl groups are attached to different carbon atoms.
Open –chain structure of glucose
On the basis of above reactions, Fisher assigned an open chain structure of glucose shown below
as structure I
The above structure of glucose is also confirmed by the cleavage reaction of glucose with
periodic acid. Five moles of periodic acid are consumed by one mole of glucose giving five
moles of formic acid and one mole of formaldehyde.

Configuration of D-Glucose
The configuration of D-glucose was proved by Emil Fisher by arguments similar to the ones
stated below.
1. Construction of four possible D-pentoses. Taking the configuration of D-glyceraldehyde
as the standard, two possible D-aldotetroses (A and B) may be constructed by adding a
CHOH just below CHO, placing OH to the right and then to the left.
Similarily, each of the two D-tetroses (A and B) gives two D-aldopentoses. Thus four possible
D-aldopentoses are:

2. D-Arabinose has configuration II or IV. Oxidation of D-arabinose with nitric acid
oxidizes the terminal CHO and CH
2
OH groups yielding two optically active dicarboxylic
acids. The forms II and IV can form two optically active diacids, while I and III can give
meso acids only that have a plane of symmetry, therefore, D-arabinose is either II or IV.
3. Configyration II confirmed for D-arabinose. D-arabinose by Killiani-Fisher synthesis
yields two epimeric aldohexoses, D-glucose and D-mannose. Thses of oxidation with
nitric acid form two optically active dicarboxylic acids. This is theoretically possible only
if D-arabinose has the configuration II and not IV.
Proceeding similarily, you will find that if D-arabinose had configuration IV, of the two
dicarboxylic acids derived from it, one would be meso and one asymmetric. Hence D-arabinose
has the configuration II.
4. Ruff degradation of D-glucose and D-mannose produces D-arabinose in each case. In ruff
degradation the CHOH below CHO is destroyed. Therefore , the configuration of the two
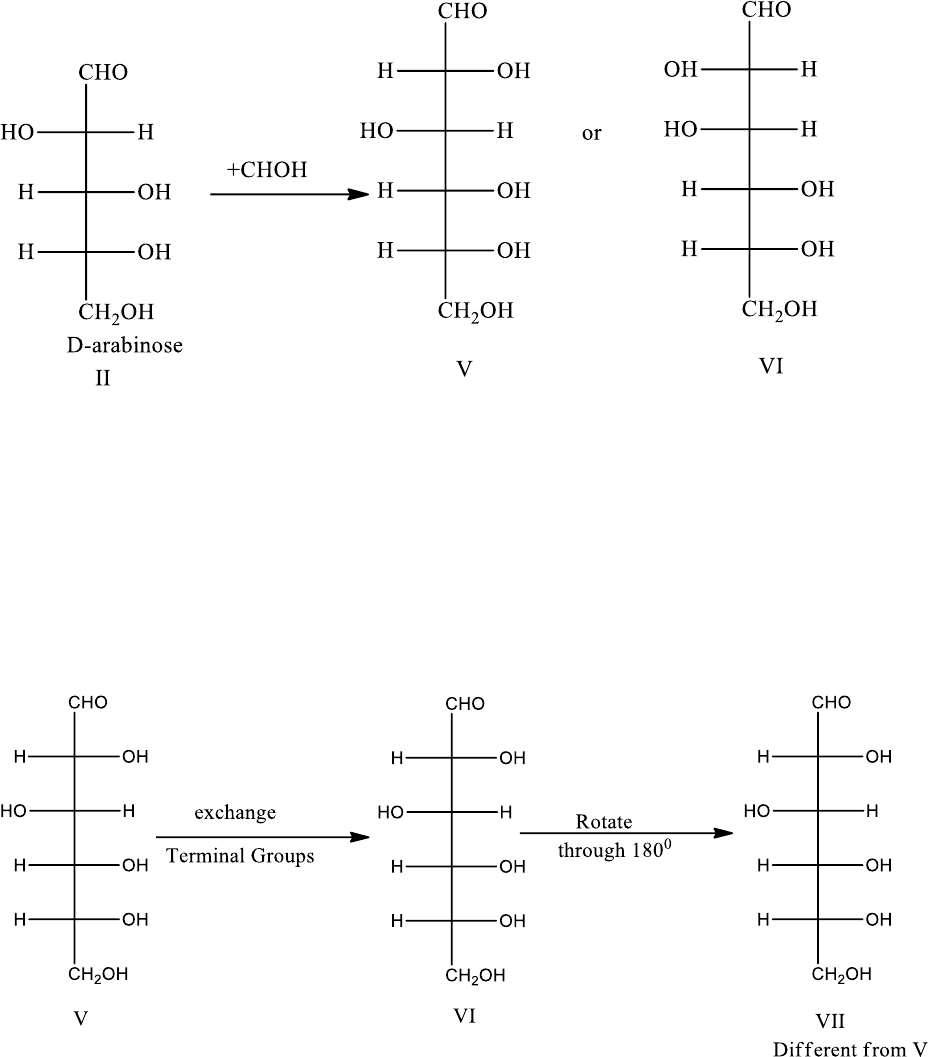
aldohexoses, D-glucose and D-mannose, can be derived by adding a new CHOH below
CHO in form II of D-arabinose.
Hence D-glucose has configuration V or VI.
5. D-Glucose and L-Glucose yield the same dicarboxylic acid. This means thattwo sugars
differ only in respect of the position of the terminal groups (CHO and CH
2
OH).
Therefore , the exchange of the terminal groups in D-glucose should be able to give a
different aldohexose (L-glucose). Let us now examine configuration formula V and VI
(one of which is D-glucose) from the angle.
If VII is rotated through 180
0
in the plane of paper, it gives an aldohexose VII, different from V.
a similar procedure with formula VI does not give rise to a different sugar.
From the above arguments it is evident that D-glucose has the configuration as shown by
the form V.
Cyclic structure of D-Glucose
The open chain structure of glucose explained most of its properties. However, it could not
explain the following facts.
1. Despite having an aldehyde group, glucose does not undergo certain characteristic
reactions of aldehyde,
(a) Glucose does not react with sodium bisulphate to form addition product.
(b) Glucose does not react with ammonia.
(c) Glucose does not give Schiff’s test and 2, 4-DNP test like other aldehydes.
2. Glucose reacts with hydroxylamine to form an oxime but glucose pentaacetate does not
react with hydroxylamine. This shows that –CHO group is not present in glucose
pentaacetate.
3. D (+)-Glucose exist in two stereoisomeric forms i.e., α- D (+)-Glucose and β- D (+)-
Glucose. These two forms are crystalline and have different m.p and optical rotations.
When glucose was crystallized from a concentrated solution at 303 K, it gave α-form of
glucose having m.p 419 K and [α]
D
= +111
0
.
On the other hand, the β-form of glucose is
obtained on crystallization of glucose from a hot saturated solution of at a temperature
above 371 K. The β-form of glucose has m.p 423 K and [α]
D
= +19.2
0
.
4. Mutarotation. When either of two forms of glucose (α- D-glucose and β- D-glucose) are
dissolved in water and allowed to stand, these get slowly converted into other form and a
equilibrium mixture of both α- D-glucose (36 %) and β- D-glucose (about 64%) is
formed.
The formation of equilibrium mixture can be explained as:
The α- D-glucose has a specific rotation of +111
0
,
while
β- D-glucose has a specific rotation of
+19.2
0
. When α-form is dissolved in water, its specific rotation falls until a constant value of
+52.5
0
is reached. On the other hand, when β-form is dissolved in water, its specific rotation
increases and becomes constant at 52.5
0
.

This spontaneous change in specific rotation of an optically active compound with time to an
equilibrium value is called mutarotation.( Latin, muto means to change).
Thus, there is an equilibrium mixture of α- and β-forms in the solution
5. Glucose forms isomeric methyl glucosides. When glucose is heated with methanol in the
presence of dry HCl, it gives two isomeric monomethyl derivatives known as α-D-
glucoside (m.p. = 438 K) and β-D-glucoside (m.p. 380 K).
These two glucosides do not reduce Fehling’s solution and also do not react with HCN or
NH
2
OH indicating that the free –CHO group is not present but it is converted to –COOH group.
Cyclic structure of Glucose
Anomers:
Glucose forms a hemiacetal between the –CHO group and the -OH group on the C
5
atom. As a
result, of cyclisation, C
1
becomes asymmetric (chiral) and the newly formed –OH group may be
either on the left or on the right in Fisher projection formulae. These results in the formation of
two isomerswhich differ in the orientation of H and –OH groups around C1 atom. These isomers
are known as α- D-glucose and β- D-glucose. The isomer having the –OH group on the right is
called α- D-glucose and one having the –OH group on the left is called β- D-glucose. Such pairs
of optical isomers which differ in the configuration only around C
1
atom are called anomers.
These two forms are not mirror image of each other, hence are not enantiomers. The C1 carbon
is known as anomeric carbon or glycosidic carbon.

The above representations are called Fisher projection formulae.
Haworth projection formulae or pyranose structures of D-Glucose.
In Haworth structures drawn with the heterocyclic oxygen in the upper right corner, the α-form
has the –OH group on C
1
pointing “down”. The β-form has the same group pointing “up”. For D-
sugars, the free –CH2OH group of an aldohexose is drawn above the plane of ring when ring
oxygen is in the upper right. The rest is the simple, the groups on the left of the Fisher projection
are up and those on the right are down in the Haworth structure.

Fructose
Fructose is another commonly known monosaccharide having the same molecular formula as
glucose. It is laevorotatory because it roatates plane polarized light towards the left. It is present
abundantly in fruits. That is why it is called fruit-sugar also.
Physical properties
1. It is sweetest of all known sugars.
2. It is readily soluble in water, sparingly soluble in alcohol and insoluble in ether.
3. It is white crystalline solid with m.p. 375 K.
4. Fresh solution of fructose has a specific rotation -133
0
.
Chemical properties of fructose
Chemical properties of fructose can be studied under the following heads:
(A) Reactions due to ketonic group
1. Reaction with HCN. Fructose reacts with HCN to form cyanohydrins.
2. Reaction with hydroxylamine. Fructose reacts with hydroxylamine to form an oxime.

3. Reduction. Fructose gives a mixture of sorbitol and mannitol on reduction with Na-Hg
and water or catalytic hydrogenation.
4. Oxidation. (i) there is no action of mild oxidizing agent like bromine water on fructose.
(ii) Strong oxidizing agents like nitric acid oxidize fructose into a mixture of
trihydroxy glutaric, glycolic and tartaric acids.
(iii) Unlike other ketones, it reduces Tollen’s reagent and Fehling solution. This is due to the
presence of traces of glucose in alkaline medium.
[B] reactions of the alcoholic group
1. Acetylation . with acetic anhydride or acetyl chloride, fructose forms penta-acetate.

2. Reaction with methyl alcohol (glucoside formation). Fructose reacts with methyl alcohol in
the presence of dry HCl gas forming methyl fructoside.
3. Reaction with metallic hydroxides (fructosate formation)
Structure of Fructose
1. elemental analysis and molecular weight determination of fructose show that it ahs the
molecular formula C
6
H
12
O
6
.
2. fructose on reduction gives sorbitol which on reduction with HI and red P gives a
mixture of n-hexane and 2-Iodohexane. This reaction indicates that six carbon atoms in fructose
are in a straight chain.
3. Fructose reacts with hydroxylamine, HCN and phenylhydrazine. It shows the presence
of _CHO or C=O group in the molecule of fructose.
4. On treatment with bromine water , no reaction takes palce. This rules out the
possibility of presence of –CHO group.
5. on oxidation with nitric acid, it gives glycollic acid and tartaric acids which contain
smaller number of carbon atoms than fructose. This shows that a ketonic group is present at
position 2. It is at this point that the molecule is broken.
Cyclic structure of D-Fructose
Fructose shows the property of mutarotation. This means that it exists in two forms α-
fructose and β-fructose which are cyclic in structure and change into each other via the open
chain structure. The cyclic and pyranose structures of α-D-fructose and β-D-fructose are
represented below:

Haworth Pyranose structure
However, when fructose is linked to glucose in a sucrose molecule, it has the furanose
structure as shown below:
7.5 MECHANISM OF OSAZONE FORMATION

Glucose and fructose react with one equivalent of phenylhydrazine, forming
phenylhydrazone. In contrast, α-hydroxy carbonyl compounds react with three equivalents of
phenylhydrazine to form bis-phenylhydrazones, commonly called osazones.
Phenylosazones crystallize readily and are useful derivatives for identifying sugars.
Mechanism: the first equivalent of phenylhydrazine forms phenylhydrazone with the aldehyde
or ketone group as expected. Phenylhydrazone the ungergoes the rearrangement , known as
Amadori rearrangement, to give α-iminoketone (IV) with the loss of aniline.

Subsequent attack of two moles of phenylhydrazine on the iminoketone (scheme-a) or on the
ketoaldehyde (scheme-b) results in the formation of osazone accompanied by the elimination of
ammonia.
The given mechanism is supported by the observation that when phenyl hydrazone
prepared by the reaction of glucose with N
15
(N
*
) labeled phenylhydrazine is treated with
ordinary phenylhydrazine, unlabelled osazone is obtained accompanied by the expulsion of
labelled ammonia.
7.6 INTERCONVERSION OF GLUCOSE AND FRUCTOSE
(a) Conversion of an aldose into an isomeric ketose. The procedure used for this purpose may be
illustrated by taking into account the conversion of glucose into fructose.
(b) Conversion of ketose into an isomeric aldose. The procedure used here may be illustrated by
taking into account the conversion of fructose into a mixture of epimeric aldoses, viz., glucose
and mannose.

7.7 CHAIN LENGTHENING AND CHAIN SHORTENING OF
ALDOSE
(a) Lengthening of aldoses: Killiani-Fisher synthesis
The aldose chains may be lengthened by one carbon atom by a procedure known as Killiani-
Fisher synthesis. Thus an aldose may be converted to the next higher member by the following
steps: (1) Formation of cyanohydrins; (2) hydrolysis of –CN to –COOH, giving aldonic acid; (3)
conversion of aldonic acid to lactone by heating; (4) reduction of lactone with sodium

borohydride, NaBH
4
, to get higher aldose. For illustration, the overall change is the creation of
an asymmetric centre at C-2 where a new CHOH has been added. Therefore their result two
aldoses with one carbon more and differing only in configuration at C-2.
Taking a specific example, D-arabinose by killiani-Fisher synthesis gives two isomeric
aldohexoses, D-glucose and D-mannose which differ only in the configuration at C-2
Such sugars which differ in configuration only at one asymmetric centre (C-2) are called
Epimers.
(b) Shortening of aldoses
(1) Ruff degradation. An aldose may be converted into a lower aldose having one carbon atom
less, i.e., the carbon chain may be shortened by Ruff degradation.

The method involves the oxidation of starting aldose into the corresponding aldonic acid.
The acid is converted into its calcium salt which is treated with Fenton’s reagent (H2O2 in
presence of Fe
+3
ion) to get the lower aldose. This method is illustrated as follows:
(2) Wohl’s degradation for chain shortening in aldoses
In this degradation, the aldose is converted into its oxime by treatment with
hydrgradazoxylamine. The oxime is treated with acetic anhydride when the oxime is dehydrated
to nitrile. The nitrile is then treated with sodium methoxide. The cyanohydrin obtained
undergoes degradation to a lower aldose. The reaction are written as under.


The osazone so formed does not undergo further amadori rearrangement. This is the reaction
with phenylhydrazine stops at this stage; thus further reaction at C-3 –OH group dost not occur.
This is because the osazone so formed, does not react further via intramolecular Amadori
rearrangement involving C-3 –OH group because of the intramolecular hydrogen bomding as
shown below:
7.8. SUMMARY
• Carbohydrates are poly hydroxy aldehydes and ketones.
• Monosaccharides containing an aldehyde group are called aldoses and those with a keto
group are called ketoses.
• Carbohydrates can also be classified as disaccharides, oligosaccharides, and
polysaccharides consist of monosaccharides linked by glycosidic bonds.
• The most abundant monosaccharide in nature is 6- carbon sugar, D-glucose. It exists as α
and β anomers with different optical rotations.
• If two monosaccharides isomers differ in configuration around one specific carbon atom
[With exception of carbonyl carbon] they are called epimers of each other.
7.9. MODEL EXAMIONATION QUESTIONS
1. Define and classify carbohydrates with suitable examples.
2. Explain kiliani-Fisher synthesis and Ruff’s degradation.
3. Explain the limitations of open chain D-glucose structure.
4. Establish the structure of glucose and fructose.

REFERENCES
1. A text book of organic chemistry, A. Bahl and B.S.Bahl, S.Chand & Compant Ltd.
2. Chemistry for degree students, R.L.madan, S.Chand & Compant Ltd.
3. Organic chemistry, Vol.III, Jagdamba singh and L.D.S Yadav, Prgati Prakashan.
4. Modern chemistry, S.P.Jauhar, Modern Publishers.
5. Organic chemistry, Francis A.Carey, Tata McGraw-Hill Company Ltd.

UNIT-8 CARBOHYDRATES-II
CONTENTS
8.1 Objectives
8.2 Introduction
8.3. Configuration of monosaccharides
8.4. Erythreo and threo distereomers conversion of glucose
8.5 ethers and esters
8.6. Determination of ring size of monosaccharides
8.7. Cyclic structure of D-glucose
8.8. Mechanism of mutarotation
8.9. General study of disaccharides
8.10. General introduction of structure of ribose and deoxyribose
8.11. General study of polysaccharides
8.12 Summary
8.13. Model examination questions
8.1 OBJECTIVES
After going through this unit you will be able to:
• Know about configuration of monosaccharides
• Discuss about Erythreo and threo distereomers conversion of glucose
• Describe ether and ester formation,
• Determination of ring size of monosaccharides,
• Cyclic structure of D-glucose

• Mechanism of mutarotation
• Types of disaccharides and their properties
• Structure of ribose and deoxyribose
• Polysaccharides and its types
8.2 INTRODUCTION
The carbohydrates are an important class of naturally occurring organic compounds.
They occur naturally in plants (where they are produced photosynthetically), when the
word "carbohydrate" was coined, it originally referred to compounds of general formula C
n(H2O) n . However, only the simple sugars or monosaccharaides fit this formula exactly. The
other types of carbohydrates, oligosaccharides,and polysaccharides, are based on
monosaccharaides units and have slightly different general formula. Carbohydrates also
called “saccharides” which means sugar in Greek.
Many commonly encountered carbohydrates are polysaccharides, including glycogen,
which is found in animals, and starch and cellulose, which occur in plant.
8.3 CONFIGURATION OF MONOSACCHARIDES
In early days of development of stereochemistry of organic compounds, it was not possible to
determine the absolute configurations. The chemists were only interested in knowing the relative
configurations. To decide about configurations, Emil Fisher in 1885 chose glyceraldehyde
(CHOCHOHCH
2
OH) as the standard substance and fixed its relative configurations arbitrarily.
This compound exists in two enantiomeric forms, as given below:
Compound I was found to be dextrorotatory and compound II was found to be laevorotatory. The
difference between configuaration of the two compounds is that in compound (I), -H is located

on the L.H.S and –OH is located on the R.H.S. of the Fisher projection formula while in
compound (II), this is in reverse order.
Configuration of other compounds was then assigned by relating their configuration to that of D-
or L-Glyceraldehyde.
In 1951 Bijvoet using x-ray crystallography established that the arbitrarily assigned
configurations of glyceraldehydes actually represented their correct absolute configurations.
Thus, if the configuration of glyceraldehydes were correct, the derived relative configurations of
other compound must also be their correct absolute configuration.
Thus D- and L- glyceraldehydes serve as reference molecule for all the monosaccharides. A
monosaccharide whose penultimate carbon (farthest chiral carbon atom from most oxidizing end
i.e, -CHO) has the same configuration as D-Glyceraldehyde has L-configuration. Similarily ,
amonosaccharide whose penultimate carbon has the same configuration as L-Glyceraldehyde has
L-configuration. This is illustrated with the help of following examples.
8.4. ERYTHRO AND THREO DIASTEROMERS CONVERSION OF
GLUCOSE
Erythro and Threo system of nomenclature is used only in aldotetroses. Aldotetrose have
two chirality centres and therefore four stereoisomers. Two of the stetreoisomers are D-sugars

and two are L-sugars. When fisher projections are drawn for stereoisomers with two adjacent
chirality centres, the pair of enantiomers with similar groups on the same side of the carbon
chain is called the erythreo enantiomers. The pair of enantiomers with similar groups on opposite
sides are called the threo enantiomers. The names of erythreo and threo pairs of enantiomers in
fact, originated from the name of aldotetroses, erythreose and threose.
Erythreose and threose are diastereomers.
8.5. ETHERS AND ESTERS
(a) Formation of ethers
It is possible to convert the –OH groups attached to carbons other than anomeric carbon into
alkyl derivatives having ordinary ether C-O-C linkages. For example methyl glucoside can be
converted into pentamehyl derivative by treatment with excess dimethyl sulphate in aqueous
sodium hydroxide. The function of sodium hydroxide is to convert hydroxyl groups into alkoxide
ions which then react with dimethyl sulphate by an S
N
2 reaction to form methyl ethers.
Since all the –OH groups are converted into-OCH3 groups, the process is called exhaustive
methylation or permethylation.
For naming these compounds, each –OCH3 group except that of glycosidic linkage is named as
an O-methyl group.
When permethylated glycoside is treated with dilute aqueous acid, the methyl glycoside bond
gets hydrolysed (since acetals are hydrolysed in acidic solution). But the other methyl groups
remain unaffected. This is because ordinary ether groups are stable in dilute aqueous acids. This
is shown as under:
The process of permethylation of glycosides followed by acidic hydrolysis of glycosidic linkage
forms an important method for decctermining the ring size of monosaccharides. This has been
illustrated in the case of cyclic structure of glucose.
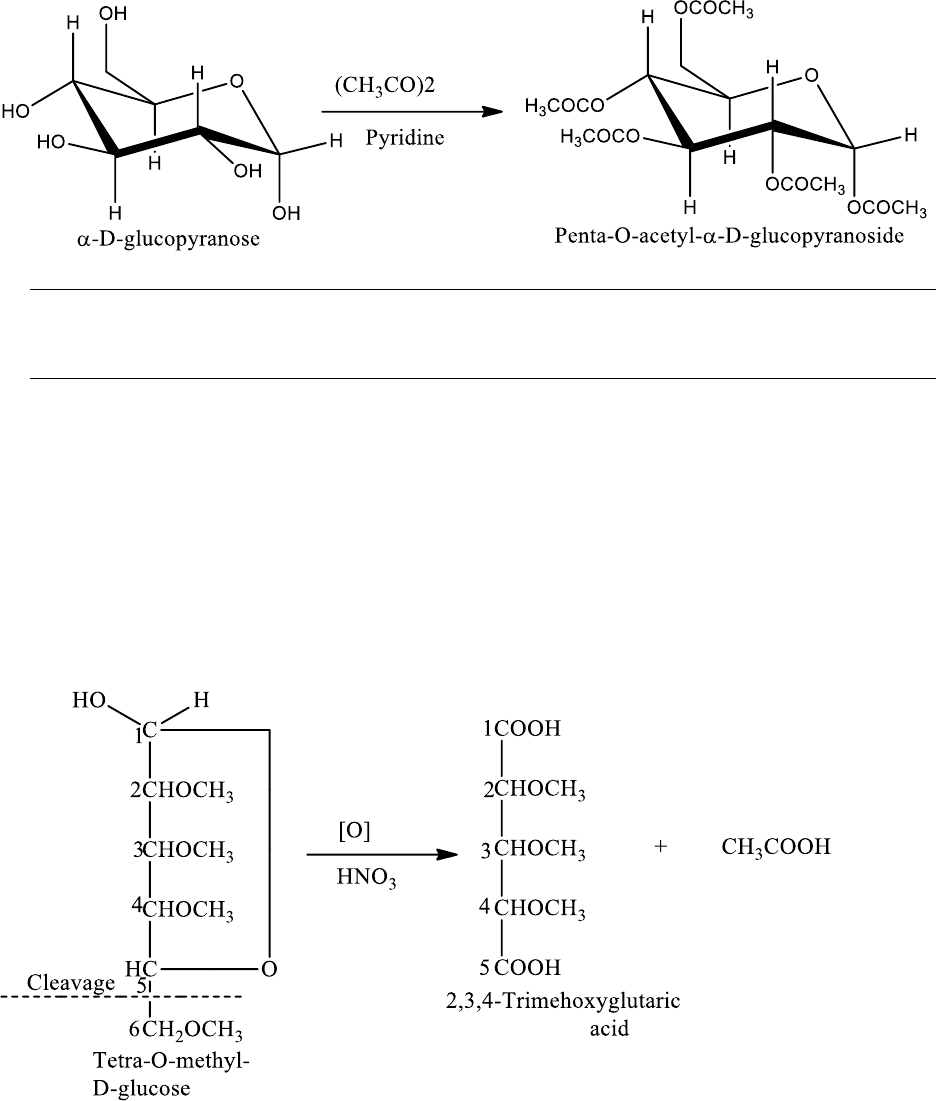
(b) Formation of esters
Monosaccharides on treatment with acetic anhydride are converted into ester derivatives which
are very useful crystalline compounds. The monosaccharide is treated with acetic anhydride and
pyridine when all the hydroxyl groups are converted to ester groups. When carried out at low
temperature (273 K), the reaction takes place stereospecifically, α-aomer gives the α-acetate and
the β-anomer gives the β-acetate. For example:
8.6. DETERMINATION OF RING SIZE OF
MONOSACCHARIDES
So far we have represented structure of cyclic hemiacetals or anomers of D-glucose as
having a ring of six me zmbers, five carbons and one oxygen. This has been proved to be correct
and a five membered ring has been ruled out.
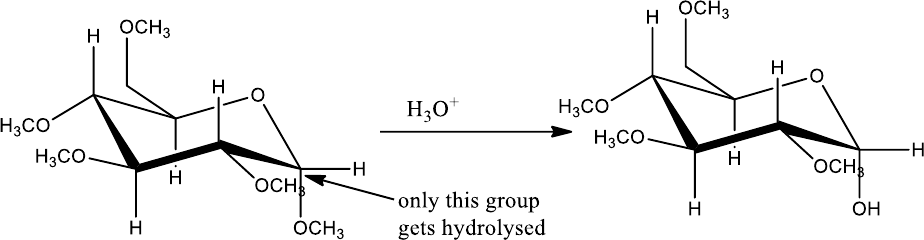
Hirst (1926) prepared tetra-O-methyl-D-glucose with dimethyl sulphate and subsequent
acid hydrolysis of the pentamethyl derivative formed. The oxidation of tetra-O-methyl-D-
glucose with nitric acid yielded trimethoxyglutaric acid.
Obviously, the two carboxylic carbons (1, 5) of the trimethoxyglutaric acid are the one’s
originally involved in ring formation. Hence, there must have existed an oxide ring between C-1
and C-5. Tracing back the reaction sequence, it stands proved that D-glucose has a six membered

ring. The presence of a 6-membered ring in D-glucose has also been confirmed by X-ray
analysis.
8.7. CYCLIC STRUCTURE OF D-GLUCOSE
The open chain structure of glucose explained most of its properties. However, it could not
explain the following facts.
6. Despite having an aldehyde group, glucose does not undergo certain characteristic
reactions of aldehyde,
(d) Glucose does not react with sodium bisulphate to form addition product.
(e) Glucose does not react with ammonia.
(f) Glucose does not give Schiff’s test and 2, 4-DNP test like other aldehydes.
7. Glucose reacts with hydroxylamine to form an oxime but glucose pentaacetate does not
react with hydroxylamine. This shows that –CHO group is not present in glucose
pentaacetate.
8. D (+)-Glucose exist in two stereoisomeric forms i.e., α- D(+)-Glucose and β- D(+)-
Glucose. These two forms are crystalline and have different m.p and optical rotations.
When glucose was crystallized from a concentrated solution at 303 K, it gave α-form of
glucose having m.p 419 K and [α]
D
= +111
0
.
On the other hand, the β-form of glucose is
obtained on crystallization of glucose from a hot saturated solution of at a temperature
above 371 K. The β-form of glucose has m.p 423 K and [α]
D
= +19.2
0
.
9. Mutarotation. When either of two forms of glucose (α- D-glucose and β- D-glucose) are
dissolved in water and allowed to stand, these get slowly converted into other form and a
equilibrium mixture of both α- D-glucose (36 %) and β- D-glucose (about 64%) is
formed.
The formation of equilibrium mixture can be explained as:
The α- D-glucose has a specific rotation of +111
0
,
while
β- D-glucose has a specific rotation of
+19.2
0
. When α-form is dissolved in water, its specific rotation falls until a constant value of
+52.5
0
is reached. On the other hand, when β-form is dissolved in water, its specific rotation
increases and becomes constant at 52.5
0
.

This spontaneous change in specific rotation of an optically active compound with time to an
equilibrium value is called mutarotation.( Latin, muto means to change).
Thus, there is an equilibrium mixture of α- and β-forms in the solution
10. Glucose forms isomeric methyl glucosides. When glucose is heated with methanol in the
presence of dry HCl, it gives two isomeric monomethyl derivatives known as α-D-
glucoside (m.p. = 438 K) and β-D-glucoside (m.p. 380 K).
These two glucosides do not reduce Fehling’s solution and also do not react with HCN or
NH
2
OH indicating that the free –CHO group is not present but it is converted to –COOH group.
Cyclic structure of Glucose
Anomers.
Glucose forms a hemiacetal between the –CHO group and the -OH group on the C
5
atom.
As a result, of cyclisation, C
1
becomes asymmetric (chiral) and the newly formed –OH group
may be either on the left or on the right in Fisher projection formulae. This result in the
formation of two isomerswhich differs in the orientation of H and –OH groups around C1 atom.
These isomers are known as α- D-glucose and β- D-glucose. The isomer having the –OH group
on the right is called α- D-glucose and one having the –OH group on the left is called β- D-
glucose. Such pairs of optical isomers which differ in the configuration only around C
1
atom are
called anomers.
These two forms are not mirror image of each other, hence are not enantiomers. The C1 carbon
is known as anomeric carbon or glycosidic carbon.

The above representations are called Fisher projection formulae.
Haworth projection formulae or pyranose structures of D-Glucose.
In Haworth structures drawn with the heterocyclic oxygen in the upper right corner, the α-form
has the –OH group on C
1
pointing “down”. The β-form has the same group pointing “up”. For D-
sugars, the free –CH
2
OH group of an aldohexose is drawn above the plane of ring when ring
oxygen is in the upper right. The rest is the simple, the groups on the left of the Fisher projection
are up and those on the right are down in the Haworth structure.
8.8. MECHANISM OF MUTAROTATION

Mutarotation occurs by opening of the ring to the free carbonyl form. The mechanism shown in
Scheme I begin as the reverse of hemiacetal (or hemiketal) formation. An 180
0
rotation about the
bond to the carbonyl group permits attack of the hydroxyl group at C-5 on the opposite face of
the carbonyl carbon. Hemiacetal formation then gives the other anomer. Mutarotation is
catalysed by both acid and base.
Thus, the easy opening and closing of hemiacetal or hemiketal linkage is responsible for
mutarotation.
8.9. GENERAL STUDY OF DISACCHARIDES
Disaccharides are the carbohydrates which on hydrolysis give two same or different
monosaccharides. Their general formula is C
12
H
22
O
11
. The important members belonging to
disaccahrides are sucrose, maltose, and lactose. On hydrolysis with dilute acids or enzymes these
give the following two molecules of monosaccharides.

In disaccharides, the two monosaccharides are joined together by acetal or glycosidic
formation. The hemiacetal OH of one monosaccharide and an OH of second monosaccharide,
dehydrate to establish the bond (called glycosidic bond) between the two monosaccharides. That
is, disaccharides are composed of two units of monosaccharides joined by glycosidic linkage.
Sucrose (Cane Sugar). Sucrose is ordinary table sugar. It is obtained from cane sugar. Sucrose
is composed of α-D-glucose and β-D-fructose unit. These units are joined by α,β-glycosidic
linkage between C-1 of glucose and C-2 of fructose unit.

Notice that in the above structure of sucrose, hemiacetal structure is missing. That is why
sucrose: (a) does not form an osazone with phenylhydrazine (b) does not reduce Tollen’s reagent
or Fehling’s solution (sucrose is a non reducing sugar) (c) does not exhibit mutarotation.
Maltose: It is obtained from starch. It is composed of two α-D-glucose units joined by a α-
glycosidic linkage between C-1 of one unit and C-4 of the other unit.
Notice that C-1 of the second glucose unit in the maltose structure is a hemiacetal carbon.
Consequently, it is in equilibrium with the open chain aldehyde form. Thus maltose can exist in
α and β forms. Since it has a potential aldehyde group, maltose shows mutarotation, forms
osazone and reduces Fehling’s solution (Maltose is a reducing sugar).
Lactose (Milk Sugar). It is found in milk of all animals. Cow’s milk contains 4-5 % and human
milk 6-7 % lactose. Lactose is composed of β-D-galactose unit and α-D-glucose unit joined by β-
D-glycosidic linkage between C-1 of the galactose and C-4 of the glucose unit.
Like maltose, lactose can also exist in α and β forms. Lactose is a reducing sugar and shows
mutarotation. It reacts with Tollen’s reagent and Fehling’s solution.
Determination of ring size of monosaccharides:

So far we have represented structure of cyclic hemiacetals or anomers of D-glucose as having a
ring of six me zmbers, five carbons and one oxygen. This has been proved to be correct and a
five membered ring has been ruled out.
Hirst (1926) prepared tetra-O-methyl-D-glucose with dimethyl sulphate and subsequent
acid hydrolysis of the pentamethyl derivative formed. The oxidation of tetra-O-methyl-D-
glucose with nitric acid yielded trimethoxyglutaric acid.
Obviously, the two carboxylic carbons (1,5) of the trimethoxyglutaric acid are the one’s
originally involved in ring formation. Hence, there must have existed an oxide ring between C-1
and C-5. Tracing back the reaction sequence, it stands proved that D-glucose has a six membered
ring. The presence of a 6-membered ring in D-glucose has also been confirmed by X-ray
analysis.
SUCROSE, Cane Sugar, (C
12
H
22
O
11
):
Sucrose is ordinary table sugar. It occurs chiefly in sugar cane and sugar beets. In smaller
amounts it is present in maple sap, honey, and several fruits.
Manufacture of Sucrose (Table Sugar):
In India and other tropical countries, the main source of sucrose is sugar cane. The modern
method for the manufacture of ‘Direct Consumption’ sugar from cane consists of the following
steps. (Fig 8.1).
(1) Juice Extraction. The crushed cane is passed through a roller mill to squeeze out juice. The
partially exhausted ‘cane mat’ emerging from the mill is passed on to a tank, called Diffuser, by
a chain conveyer. Here the maximum extraction of sucrose is done by washing with hot water
and dilute juice on counter-current principle. This technique gets sugar extraction upto 98%. The
cellulosic material discharged from the diffuser is called Bagasse and is used as fuel under
boilers.
(2) Juice Purification: The raw juice contains 14-25% sucrose and much impurity such as
organic acids, inorganic salts. Proteins and colouring matter. It is purified by the operations listed
below:
(i) Defecation: The juice is heated with high pressure steam and treated with 2-3 % lime in a
steel tank. This operation called defecation throws out organic acids as insoluble calcium salts,
coagulated protein and colouring matter. The precipitate is removed by filtration.
(ii) Carbonation: Through the filtered juice is then CO
2
. This operation known as carbonation,
removes the excess, of lime as calcium carbonate which entraps colouring matter, colloidal and
some inorganic salts. The ‘mud’ that settles is separated by filtration.
(iii) Decolorisation: In India, the clarified juice is decolorized by treating with SO
2
. This
operation called Sulphitation while it bleaches the brown colour of the juice, completes the
neutralization of lime. The insoluble calcium sulphite is removed by filtration.
(3) Concentration and Crystallisation: The clear solution is then concentrated by boiling under
reduced pressure in Multiple Effect Evaporators. In these, the steam produced in the first
evaporator is used to boil the juice in the second maintained at a lower pressure; the second
being used to boil the juice in the third kept at a still lower pressure; and son on.
The concentrated juice is finally passed to the Vacuum Pan where further evaporation
reduces the water content to 6-8%. Here partial separation of crystals takes place. The mixture of

syrup and crystals, known as Massecuite, is then discharged into a large tank, the Crystallising
Tank, fitted with cooling pipes. The crystals grow and form a thick crop.
(4) Separation of Crystals by Centriguagation, and Drying. The massecuite is then sent to
centrifuges wherby sugar crystals are separated from the syrup. The crystals are here sprinkled
with a little water to wash any syrup sticking to the surface. The wet sugar is dried by passing
down a rotating drum with stream of hot air flowing counter-current to it. The residual mother
liquor, from which the crystals have been removed, is called molasses. In India, it is valuble raw
material for alcohol manufacture by fermentation.
Fig 8.1. sugar manufacture (Flow sheet)

Properties of Cane Sugar, C
12
H
22
O
11
:
1. It is colourless, crystalline substance, sweet in taste. It is very soluble in water and the solution
is dextrorotatory [α]
D
= +66.5.
2. Effect of heat. Sucrose on heating slowly and carefully melts and then if allowed to cool, it
solidifies to pale-yellow glassy mass called ‘barley sugar’.
When heated to 473K, it loses water to form a brown amorphous mass called caramel. On
strong heating it chars to almost pure carbon giving characteristic smell of burnt sugar.
3. Hydrolysis or Inversion of Sucrose (Sugar). Sucrose when boiled with mineral acids, or by the
enzyme invertase, yields an equimolar mixture of glucose and fructose.
Sucrose is dextrorotatory and on hydrolysis produces dextrorotatory glucose and
laevorotatory fructose. With greater laevorotation of fructose the mixture is laevorotatory. Thus,
there is a change (inversion) in the direction of rotation of the reaction mixture from dextro to
laevo. This phenomenon is called inversion and the enzyme which brings about this inversion is
called invertase.
4. Formation of Sucrosates: Sucrose solution reacts with calcium, barium and strontium
hydroxides to form sucrosates.
The sucrosate decomposes when carbon dioxide is passed in the solution.

5. Action of nitric acid: Concentrated nitric acid oxidizes cane sugar to oxalic acid.
6. Fermentation: Fermentation of Sucrose is brought about by yeast when the enzymes
invertase hydrolysed sucrose to glucose and fructose and zymase converts them to ethyl alcohol.
8.10. GENERAL INTRODUCTION OF STRUCTURE OF RIBOSE
AND DEOXYRIBOSE
Ribose and deoxyribose are two well known aldopentoses. Their structures are discussed as
under.
Structure of D-(+)- Ribose:
D-(+)- Ribose occurs naturally in plant nucleic acids and in liver and pancreas nucleic acids. It
gives properties similar to glucose.
Ribose has the molecular formula (C
5
H
10
O
5
) and shows the presence of an aldehyde
group, four hydroxyl groups (one primary and three secondary) and a straight chain of carbon
atoms. Therefore, it was assigned an open chain formula as given below:
5
CH
2
OH-
4
CHOH-
3
CHOH-
2
CHOH-
1
CHO
The configuaration of D-ribose has been established as follows.

As in the case of glucose, D-ribose is now assigned a ring structure and is known to exist both in
furanose and pyranose forms as depicted below:
Pyranose form is more stable than the furanose form. Equilibrium mixture of ribose
contains 56% β-D-ribopyranose 20% α-D-ribopyranose, 18% β-D-ribofuranose and 6% α-D-
ribofuranose. In RNA ribose is present in furanose form.
Structure of deoxyribose:

In this aldopentose the hydroxyl group at C-2 of ribose has been replaced by hydrogen.
That is why it is named as deoxyribose. It is fundamental constituent of deoxyribonucleic acid
(DNA).
The structure of D-2-deoxyribose is derived from that of D-ribose and may be
represented in the open chain and ring forms as follows.
8.11 GENERAL STUDY OF POLYSACCHARIDES
These are neutral polymeric compounds in which hundreds or even thousands of
monosaccharide units are joined by glycosidic linkages. They have the general formula
(C
5
H
10
O
5
)
n
, where n has very large value. They are colourless, tasteless and are insoluble in
water. They play very important role in plant and animal life as food storage and structural role.
They are usually made up of pentoses or hexoses. The important polysaccharides are cellulose,
starch, glycogen and dextrins.
Starch:
Starch is most widely distributed in vegetable kingdom. In nature, it is transformed into
complex polysaccharides like gum and cellulose and into simpler mono and disaccharides by
enzymes working in vegetable kingdom. Its rich sources are potatoes, wheat, maize, rice, barley
and arrow root. It is interesting to note that no two sources give identical starch.
Physical properties:

It is a white, amorphous substance with no taste or smell. It is insoluble in water but when starch
is added to boiling water the granules swell and burst forming colloidal, translucent suspension.
Chemical properties of starch:
(i) When heated to a temperature between 200-250
0
it changes into dextrin. At higher
temperature charring takes place.
(ii) Starch, when boiled with dilute acids, yielded ultimately glucose
When hydrolysed with enzyme diastase, maltose is obtained.
(iii) Starch solution gives a blue colour with a drop of iodine solution. The blue Colour
disappears on heating and reappears on cooling. In fact it is the amylase that gives colour with
iodine; the amylopectin gives a red brown colour with iodine.
Starch is a non reducing saccharide. It does not reduce Fehling’s solution or Tollen’s reagent. It
also does not form an osazone indicating that all hemiacetal hydroxyl groups of glucose units
(C
1
) are not free but are linked with glycosidic linkages.
Starch is polymer of α-D-glucose and consists of two components (15-20%) amylase and 80-
85%) amylopectin.
(i) Amylose. It is white soluble farction. It is linear polymer of α-D-glucose. It contains about
200-1000 α-D-glucose units which are linked to one another through α-glycosidic linkage
involving C-1 of one glucose and C-4 of the next as shown below.

Its molecular mass can range from 10, 0000 to 500,000.
(ii) Amylopectin. It is water insoluble fraction. It is a highly branched chain polymer which does
not give blue colour with iodine. It consists of a large number of short chains of 25-30 D-glucose
units. In this case the main chain involves α-linkages between C-1 of one α-D-glucose unit and
C-4 of the other. The C-1 of terminal glucose in each chain is further linked to C-6 of the other
glucose unit in the next chain through C-1-C-6 α-linkage. This gives highly branched structure.
Starch is used as the principal food storage of glucose energy. It is hydrolysed by enzyme
amylase present in saliva. The end product of glucose which is an essential nutrient.
Cellulose

Cellulose is the main structural material of trees and other plants. Wood is 50% cellulose,
while cotton wool is almost pure cellulose. Other sources of cellulose are straw, corncobs,
bagasse, and similar agriculture wastes.
Manufacture: Cotton wool is about 97% cellulose. It is ready for use after washing away the
waxes and fats associated with it. The cellulose required for making paper is obtained from
wood. Lignin and resinous substances present along with cellulose are removed by digesting the
wood chips under pressure with a solution calcium hydrogen sulphite. The cellulose separates as
insoluble fibres which are washed with water, bleached and dried.
Structure. Cellulose is a straight chain polysaccharide composed of D-glucose units. These units
are joined by β-glycosidic linkages between C-1 of one glucose unit and C-4 of the next glucose
unit. The number of D-glucose units in cellulose ranges from 300-2500.
Properties: Cellulose is a colourless amorphous solid having no m.p. it decomposes om strong
heating. It is insoluble in water and most organic solvents. However, it dissolves S. reagent
which is an ammonical solution of cupric hydroxide.
Hydrolysis: Cellulose when hydrolysed by heating with dilute acids, gives D-glucose.
Cellobiose is formed in case of incomplete hydrolysis.
The cattle, goats, and other ruminants have digestive enzymes (Cellulases) capable of
hydrolyzing cellulose into glucose. Consequently, these can feed directly on cellulose. Man and
many other mammals lack the necessary enzymes in their digestive tract, and they cannot use
cellulose as foodstuff.

8.12. SUMMARY
• Glyceraldehydes are the simplest carbohydrate and it serves as a reference
molecule to write the configuration (D and L) of all other monosaccharides.
• The pair of enantiomers with similar groups on the same side of the carbon chain
is called the erythreo enantiomers while pair of enantiomers with similar groups
on opposite sides is called the threo enantiomers.
• Mutarotation is defined as the interconversion of and β anomeric forms with the
change in the optical rotation.
• Disaccharides are the carbohydrates which on hydrolysis give two same or
different monosaccharides.
• Disaccharides are composed of two units of monosaccharides joined by glycosidic
linkage.
• Polysaccharides are neutral polymeric compounds in which hundreds or even
thousands of monosaccharide units are joined by glycosidic linkages.
8.13. MODEL EXAMINATION QUESTIONS
1. How will you convert glucose into fructose?
2. Discuss the mechanism of mutarotation.
3. How is sucrose manufactured from sugar cane.
4. Discuss the structure of sucrose, lactose and maltose.
5. Write a short note on polysaccharides
REFERENCES
1. A text book of organic chemistry, A. Bahl and B.S.Bahl, S.Chand & Compant Ltd.
2. Chemistry for degree students, R.L.madan, S.Chand & Compant Ltd.
3. Organic chemistry, Vol.III, Jagdamba singh and L.D.S Yadav, Prgati Prakashan.
4. Modern chemistry, S.P.Jauhar, Modern Publishers.
5. Organic chemistry, Francis A.Carey, Tata McGraw-Hill Company Ltd.
UNIT WRITER- Dr. Devendra Singh Dhami ,Department of Chemistry SSJ Campus, Almora
