
Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands
An agency of the European Union
Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us
Send us a question Go to www.ema.europa.eu/contact
Telephone +31 (0)88 781 6000
© European Medicines Agency, 2022. Reproduction is authorised provided the source is acknowledged.
8 July 2022
EMA/285848/2020
Information Management
Product Management Service (PMS) - Implementation of
International Organization for Standardization (ISO)
standards for the identification of medicinal products
(IDMP) in Europe
Chapter 2: Data elements for the electronic submission of information on
medicinal products for human use
Version 2.1.1.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 2/231
Table of contents
Summary of changes ................................................................................... 7
Glossary .................................................................................................... 12
Scope of this guidance ............................................................................... 14
Medicinal products in scope ....................................................................... 14
Submission of medicinal products authorised in EEA countries outside the EU ................... 15
Submission of information on medicinal products with valid marketing authorisation in the
territory of Northern Ireland ....................................................................................... 15
Submission of medicinal products authorised under mutual recognition or decentralised
procedure in Liechtenstein ......................................................................................... 15
Marketing authorisations granted by the Swiss authorities and recognised by Liechtenstein 16
Submission of medicinal product data using FHIR ..................................... 16
Identifiers and defining characteristics of a medicinal product entry in PMS
.................................................................................................................. 17
Product Management Service Identifier (PMS ID) .......................................................... 18
Medicinal Product Identifier (MPID) ............................................................................. 19
Packaged Medicinal Product Identifier (PCID) ............................................................... 21
Relationship between PMS ID and ISO IDMP standard 11615 Medicinal Product Identifier
(MPID) and Packaged Medicinal Product Identifier (PCID) .............................................. 22
Imatinib Company A 25 mg tablets ............................................................................. 24
Imatinib company A 50 mg tablets .............................................................................. 25
Access to identifiers .................................................................................................. 26
User guidance ............................................................................................ 27
Provenance ............................................................................................... 31
1. Medicinal product .................................................................................. 34
1.1. Product Management Service Identifier (PMS ID) ................................................... 35
1.2. Medicinal Product Identifier (MPID) ....................................................................... 35
1.3. Domain ............................................................................................................. 37
1.4. Type ................................................................................................................. 37
1.5. (Authorised) pharmaceutical form ........................................................................ 38
1.6. Combined pharmaceutical dose form .................................................................... 39
1.7. Legal status of supply ......................................................................................... 40
1.8. Additional monitoring indicator ............................................................................. 41
1.9. Orphan designation ............................................................................................ 42
1.9.1. Regulatory authorisation type ........................................................................... 43
1.9.2. Orphan designation status ................................................................................ 44
1.9.3. Orphan designation number .............................................................................. 44
1.9.4. Orphan designation status date ......................................................................... 45
1.9.5. Market exclusivity start date ............................................................................. 45
1.10. Paediatric use indicator ..................................................................................... 46
1.11. Full indication text ............................................................................................ 47
1.11.1. Language ...................................................................................................... 49
1.12. EURD ID .......................................................................................................... 49

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 3/231
1.13. Product classification ......................................................................................... 50
1.13.1. xEVMPD product type information .................................................................... 51
1.13.2. Legal basis .................................................................................................... 51
1.13.3. ATC code(s) .................................................................................................. 52
1.13.4. Medicinal product category.............................................................................. 54
1.13.5. Genetically Modified Organisms (GMOs) ............................................................ 55
1.14. Medicinal product name ..................................................................................... 55
1.14.1. Full name ..................................................................................................... 56
1.14.2. Country/Language ......................................................................................... 57
1.14.3. (Medicinal product name) name part(s) ............................................................ 60
1.15. (Pharmacovigilance) master file.......................................................................... 71
1.15.1. File type ....................................................................................................... 72
1.15.2. File code ....................................................................................................... 72
1.16. Contact (QPPV) ................................................................................................ 74
1.16.1. Identifier ...................................................................................................... 75
1.16.2. Role ............................................................................................................. 76
1.17. Pharmacovigilance enquiry information ............................................................... 77
1.17.1. Email address ................................................................................................ 77
1.17.2. Phone number ............................................................................................... 78
1.17.3. Role ............................................................................................................. 79
1.18. Attached document ........................................................................................... 79
1.18.1. Master (Attached document) Identifier ............................................................. 83
1.18.2. Alternative (Attached document) Identifier ........................................................ 84
1.18.3. (Attached document) Type .............................................................................. 86
1.18.4. (Attached document) Effective Date ................................................................. 87
1.18.5. (Attached document) Language ....................................................................... 87
1.18.6. URL value (New) ............................................................................................ 88
1.18.7. (Attached document) Status (New) .................................................................. 88
1.19. Product cross-reference ..................................................................................... 89
1.19.1. Product cross-reference type ........................................................................... 90
1.19.2. Product cross-reference resource identifier ........................................................ 91
1.20. Manufacturing business operation ....................................................................... 91
1.20.1. Manufacturer ................................................................................................. 92
1.20.2. Operation type .............................................................................................. 93
1.20.3. Manufacturing operation start date .................................................................. 94
1.20.4. Manufacturing operation end date .................................................................... 94
1.20.5. Confidentiality indicator .................................................................................. 95
1.20.6. Manufacturing authorisation reference number .................................................. 96
1.20.7. Effective date ................................................................................................ 97
1.20.8. (Manufacturing business operation) Medicines Regulatory Agency Organisation ..... 98
2. Marketing authorisation information ................................................... 100
2.1. Regulatory authorisation type ............................................................................ 101
2.2. Marketing authorisation number ......................................................................... 101
2.3. Country ........................................................................................................... 103
2.4. Authorisation status .......................................................................................... 104
2.5. Authorisation status date .................................................................................. 105

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 4/231
2.6. Date of first authorisation .................................................................................. 105
2.7. International birth date ..................................................................................... 107
2.8. Marketing authorisation holder (organisation) ...................................................... 111
2.9. (Marketing authorisation) Regulator .................................................................... 112
2.10. Marketing authorisation procedure .................................................................... 113
2.10.1. Procedure Identifier ..................................................................................... 113
2.10.2. Procedure type – Medicines approval system ................................................... 115
2.10.3. Procedure start date ..................................................................................... 116
2.10.4. Procedure end date ...................................................................................... 117
2.10.5. Regulatory application .................................................................................. 117
3. Therapeutic (product) indication ......................................................... 121
3.1. Indication as "Disease/Symptom/Procedure" ....................................................... 121
3.2. Co-morbidity ................................................................................................... 122
3.3. Intended effect ................................................................................................ 124
4. Packaged medicinal product ................................................................ 127
4.1. Packaged Medicinal Product Identifier (PCID) ....................................................... 130
4.2. Package description .......................................................................................... 131
4.2.1. Language ..................................................................................................... 132
4.3. Manufacturer (New) .......................................................................................... 132
4.4. Pack size ......................................................................................................... 133
4.4.1. Quantity operator (New)................................................................................. 134
4.5. Legal status of supply ....................................................................................... 135
4.6. Marketing status .............................................................................................. 136
4.6.1. Country ........................................................................................................ 137
4.6.2. Marketing status ............................................................................................ 137
4.6.3. (Marketing status) start date .......................................................................... 138
4.6.4. (Marketing status) end date ............................................................................ 138
4.6.5. Risk of supply shortage .................................................................................. 139
4.6.6. Risk of supply shortage comment .................................................................... 140
4.6.7. Status reason ................................................................................................ 140
4.7. Marketing authorisation (Package level) .............................................................. 142
4.7.1. Regulatory authorisation type ......................................................................... 143
4.7.2. Marketing authorisation number (Package level) ................................................ 143
4.7.3. Country ........................................................................................................ 144
4.7.4. Authorisation status ....................................................................................... 145
4.7.5. Authorisation status date (Package level) ......................................................... 145
4.8. Package item (container) .................................................................................. 146
4.8.1. Package item (container) type ......................................................................... 148
4.8.2. Package item reference(s) .............................................................................. 149
4.8.3. Manufactured item reference(s)....................................................................... 150
4.8.4. Device reference(s) ....................................................................................... 150
4.8.5. Package item (container) quantity ................................................................... 151
4.8.6. Data carrier identifier ..................................................................................... 153
4.8.7. Material ........................................................................................................ 154
4.9. Package (component) ....................................................................................... 155

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 5/231
4.9.1. Component type ............................................................................................ 156
4.9.2. Component material ...................................................................................... 157
4.10. Medical device ................................................................................................ 157
4.10.1. Type of medical device used in combination with medicinal product ................... 159
4.10.2. Medical device type ...................................................................................... 159
4.10.3. Medical device identification .......................................................................... 160
4.10.4. Medical device trade name ............................................................................ 161
4.10.5. Medical device quantity ................................................................................ 162
4.10.6. Medical device description (New) ................................................................... 163
4.10.7. Medical device description of intended purpose (New) ...................................... 164
4.10.8. Medical device classification (New) ................................................................. 166
4.10.9. Medical device manufacturer (New)................................................................ 167
4.11. Manufactured item .......................................................................................... 167
4.11.1. Unit of presentation ..................................................................................... 168
4.11.2. Manufactured item quantity .......................................................................... 169
4.11.3. Manufactured dose form ............................................................................... 171
4.11.4. Ingredient ................................................................................................... 172
4.11.5. Manufactured item description ....................................................................... 173
4.12. Shelf life / Storage .......................................................................................... 174
4.12.1. Shelf life type .............................................................................................. 176
4.12.2. Shelf life time period and units ...................................................................... 176
4.12.3. Special precautions for storage ...................................................................... 177
5. Ingredient ........................................................................................... 179
5.1. Ingredient role ................................................................................................. 180
5.2. Origin of the substance ..................................................................................... 180
5.3. Composition grouping description ....................................................................... 181
5.4. Manufacturer ................................................................................................... 181
5.5. Substance ....................................................................................................... 182
5.5.1. Substance .................................................................................................... 182
5.5.2. Substance strength (quantitative composition) .................................................. 184
5.5.3. Substance reference strength (quantitative composition) .................................... 192
5.5.4. (Certificate) master file .................................................................................. 199
6. Pharmaceutical product ....................................................................... 203
6.1. Pharmaceutical product description ..................................................................... 206
6.1.1. Language ..................................................................................................... 207
6.2. Administrable dose form ................................................................................... 208
6.3. Unit of presentation .......................................................................................... 208
6.4. Ingredient ....................................................................................................... 209
6.5. Device ............................................................................................................ 209
6.6. Route of administration ..................................................................................... 210
7. Annex I - PMS ID, MPIDs and PCIDs relationship during lifecycle of
medicinal products – Examples ............................................................... 211
7.1. MPIDs/PCIDs examples* CAPs ........................................................................... 211
7.2. MPIDs/PCIDs examples* MRP/DCP ..................................................................... 215

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 7/231
Summary of changes
Following the publication of version 2.1 in June 2021, the content of this document was amended to
contain the following changes:
• General updates to improve the quality of the user guidance;
• Reference newly created RMS lists and updated existing lists to support PMS data entry;
• Contain updated technical information on conformance, data type, value, conformance, ISO/FHIR
elements name/paths and FHIR Complementary Information across the guidance, where
applicable;
• Contain updated information in Annex II - Common/European Union (EU) and national data set to
be in alignment with the contents of the relevant Chapter 2;
• Include new paragraph Submission of information on medicinal products with valid marketing
authorisation in the territory of Northern Ireland
• The below listed sections now include updated information:
− Identifiers and defining characteristics of a medicinal product entry in PMS
− Product Management Service Identifier (PMS ID),
− Medicinal Product Identifier (MPID)
− Relationship between PMS ID and ISO IDMP standard 11615 Medicinal Product Identifier
(MPID) and Packaged Medicinal Product Identifier (PCID)
− User guidance
− Reason
− 1.2. Medicinal Product Identifier (MPID)
− 1.3. Domain
− 1.4. Type
− 1.5. (Authorised) pharmaceutical form
− 1.6. Combined pharmaceutical dose form
− 1.7. Legal status of supply
− 1.9.1. Regulatory authorisation type
− 1.9.2. Orphan designation status
− 1.9.3. Orphan designation number
− 1.9.4. Orphan designation status date
− 1.9.5. Market exclusivity start date
− 1.10. Paediatric use indicator
− 1.11.1. Language
− 1.13. Product classification
− 1.13.1. xEVMPD product type information

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 8/231
− 1.13.2. 1.10. Legal basis
− 1.13.3. ATC code(s)
− 1.13.4. Medicinal product category
− 1.13.5. Genetically Modified Organisms (GMOs)
− 1.14. Medicinal product name
− 1.14.2. Country/Language
− 1.14.2.1. Country
− 1.14.2.2. Language
− 1.14.3.1. Name part type
− 1.14.3.2. Name part text
− 1.15.1. File type
− 1.15.2. File code
− 1.16.2. Role
− 1.17.1. Email address
− 1.17.3. Role
− 1.18. Attached document
− 1.18.1. Master (Attached document) Identifier
− 1.18.1.1. Identifier value
− 1.18.1.2. Identifier system
− 1.18.2.1. Identifier value
− 1.18.2.2. Identifier system
− 1.18.3. (Attached document) Type
− 1.18.4. (Attached document) Effective Date
− 1.18.5. (Attached document) Language
− 1.19.1. Product cross-reference type
− 1.19.2. Product cross-reference resource identifier
− 1.20.1. Manufacturer
− 1.20.2. Operation type
− 1.20.3. Manufacturing operation start date
− 1.20.4. Manufacturing operation end date
− 1.20.5. Confidentiality indicator
− 1.20.6. Manufacturing authorisation reference number
− 1.20.7. Effective date

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 9/231
− 1.20.8. (Manufacturing business operation) Medicines Regulatory Agency Organisation
− 2.1. Regulatory authorisation type
− 2.3. Country
− 2.4. Authorisation status
− 2.9. (Marketing authorisation) Regulator
− 2.10.2. Procedure type – Medicines approval system
− 2.10.5.2. Regulatory application type
− 3.1. Indication as "Disease/Symptom/Procedure"
− 3.2. Co-morbidity
− 3.3. Intended effect
− 4.2.1. Language
− 4.4. Pack size
− 4.5. Legal status of supply
− 4.6.1. Country
− 4.6.2. Marketing status
− 4.6.5. Risk of supply shortage
− 4.6.7.1. Reason
− 4.7.1. Regulatory authorisation type
− 4.7.3. Country
− 4.7.4. Authorisation status
− 4.8. Package item (container)
− 4.8.1. Package item (container) type
− 4.8.5. Package item (container) quantity
− 4.8.6. Data carrier identifier
− 4.8.7. Material
− 4.9. Package (component)
− 4.9.1. Component type
− 4.9.2. Component material
− 4.10. Medical device
− 4.10.1. Type of medical device used in combination with medicinal product
− 4.10.2. Medical device type
− 4.10.3. Medical device identification
− 4.10.4. Medical device trade name

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 10/231
− 4.10.5. Medical device quantity
− 4.11.1. Unit of presentation
− 4.11.2. Manufactured item quantity
− 4.11.3. Manufactured dose form
− 4.11.5. Manufactured item description
− 4.11.5.1. Language
− 4.12. Shelf life / Storage
− 4.12.1. Shelf life type
− 4.12.2. Shelf life time period and units
− 4.12.3. Special precautions for storage
− 5.1. Ingredient role
− 5.2. Origin of the substance
− 5.4. Manufacturer
− 5.5. Substance
− 5.5.1. Substance
− 5.5.2. Substance strength (quantitative composition)
− 5.5.2.2. Strength (presentation)
− 5.5.2.2.1. Quantity operator
− 5.5.2.2.2. Strength (presentation single value or low limit)
− 5.5.2.2.3. Strength (presentation high limit)
− 5.5.2.3. Strength (concentration)
− 5.5.2.3.1. Quantity operator
− 5.5.2.3.2. Strength (concentration single value or low limit)
− 5.5.2.3.3. Strength (concentration high limit)
− 5.5.3. Substance reference strength (quantitative composition)
− 5.5.3.1. Reference substance
− 5.5.3.2. Quantity operator
− 5.5.3.3. Reference strength (Presentation)
− 5.5.3.3.1. Quantity operator
− 5.5.3.3.2. Reference strength (Presentation single value or low limit)
− 5.5.3.3.3. Reference strength (Presentation high limit)
− 5.5.3.4. Reference strength (Concentration)
− 5.5.3.4.1. Quantity operator

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 11/231
− 5.5.3.4.2. Reference strength (Concentration single value or low limit)
− 5.5.4.1. File type
− 5.5.4.2.1. File identifier type
− 5.5.4.2.2. File Identifier
− 5.5.4.3. Submission date
− 5.5.4.4. Date of last update
− 5.5.4.5. Manufacturer
− 6.1. Pharmaceutical product description
− 6.1.1. Language
− 6.2. Administrable dose form
− 6.3. Unit of presentation
− 6.5. Device
− 6.6. Route of administration
− 8. Annex II - Common/European Union (EU) and national data set
• The below listed sections were inserted as new:
− 1.18.6. URL value (New)
− 1.18.7. (Attached document) Status (New)
− 4.3. Manufacturer (New)
− 4.8.5.1. Quantity operator (New)
− 4.8.6.1. Identifier value (New)
− 4.8.6.2. Identifier system (New)
− 4.10.5.1. Quantity operator (New)
− 4.10.6. Medical device description (New)
− 4.10.6.1. Language (New)
− 4.10.7. Medical device description of intended purpose (New)
− 4.10.7.1. Language (New)
− 4.10.8. Medical device classification (New)
− 4.10.9. Medical device manufacturer (New)
− 4.11.2.1. Quantity operator (New)

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 12/231
Glossary
ASMF: Active Substance Master File
ATC code: Anatomical Therapeutic Chemical code
ATMP: Advanced therapy medicinal product
CAPs: Centralised Authorised Products.
CE: Certification mark that indicates conformity with health, safety, and environmental protection
standards for products sold within the European Economic Area (EEA)
CEP: Certificate of Suitability
DCP: Decentralised procedure
eAF: electronic Application Form
EC: European Commission
EEA: European Economic Area
EMA: European Medicines Agency
EMEA: European Medicines Evaluation Agency
EMVS: European Medicines Verification System
EU: European Union
EUDAMED: European database on medical devices
EURD: The European Union reference dates (EURD)
FHIR: Fast Healthcare Interoperability Resources
GMO: genetically modified organism
GMP: Good distribution-practice certificates
HMA: Heads of Medicines Agencies
IBD: International birth date
ID: Identifier
IDMP: Identification of Medicinal Products
IG: Implementation guide
IS/LI/NO: Iceland, Liechtenstein, Norway
ISO: International Organization for Standardization
LOC ID: Location Identity
MA: Marketing Authorisation
MAA: Marketing Authorisation Application
MAH: Marketing Authorisation Holder

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 13/231
MFL EV: Master File Location EudraVigilance
MPID: Medicinal Product Identifier
MRP: Mutual recognition procedure
NAPs: Nationally Authorised Products
NCA: National competent authority
OMS: Organisations Management Service
ORG ID: Organisation identity
PCID: Packaged Medicinal Product Identifier
PL: Package leaflet
PMF: Plasma Master File
PMS: Product Management Service
PSMF: Pharmacovigilance system master file
PSURs: Periodic Safety Update reports.
PSUSA Periodic Safety Update Report (PSUR) single assessment (PSUSA)
RMS: Referentials Management Service
SMS: Substance Management Service (SMS)
SPOR: Substances Products Organisations Referentials
TSEs: transmissible Spongiform Encephalopathies
VAMF: Vaccine Antigen Master File
XEVMPD: eXtended EudraVigilance medicinal product dictionary (XEVMPD)
XEVPRM: Extended EudraVigilance Medicinal Product Report Message

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 14/231
Scope of this guidance
This document provides detailed guidance on the data elements and associated business rules
applicable to:
• the submission of information of authorised medicinal products for human use;
1
• the maintenance of authorised medicinal product data previously submitted.
to the Product Management Service (PMS) only in accordance with the International Organisation for
Standardisation (ISO), Identification of Medicinal Products (IDMP).
ISO IDMP standards specify the use of standardised definitions for the identification and description of
medicinal products for human use.
ISO IDMP distinguishes between Authorised Medicinal Product and Investigational Medicinal Product.
This guidance covers only aspects relevant to the Authorised medicinal product parts of the standard.
The use of ISO IDMP is required in accordance with Articles 25 and 26 of Commission Implementing
Regulation (EU) No 520/2012. These provisions mandate Member States, marketing authorisation
holders and the European Medicines Agency (EMA) to use ISO IDMP standards for the exchange and
communication of information on medicinal products.
Medicinal products in scope
This guidance applies to all authorised products that fall under the scope of Article 57(2) of Regulation
(EC) No 726/2004, as amended by Regulation (EU) 1235/2010 and Regulation (EU) 1027/2012, which
mandates marketing authorisation holders to submit and maintain product information electronically on
all authorised medicinal products for human use.
Medicinal products falling out of scope of Article 57(2) of Regulation (EC) No 726/2004 legal obligations
include:
• investigational medicinal products;
• products for which the marketing authorisation is not valid;
• traditional use registration for herbal medicinal products (Article 16a of Directive 2001/83/EC);
• simplified registration for homeopathic medicinal products (Article 14 of Directive 2001/83/EC);
• medicinal products within the scope of Article 5 of Directive 2001/83/EC i.e., 'Named patient use'
falling under Article 5(1) and 'EU Distribution Procedure' under Article 5(2);
• parallel distribution/parallel import of medicinal products (Article 76(3) and (4) of Directive
2001/83/EC);
• medicinal products authorised outside the European Economic Area (EEA) or following a non-EU
procedure;
• extemporaneous Medicinal products (e.g., medicinal products prepared in a pharmacy based on a
medical prescription such as pharmacy preparations);
• intermediate products intended for subsequent processing by an authorised manufacturer.
1
The term “Authorised” refers to concept of authorized and registered medicinal products as defined in ISO IDMP.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 15/231
Medicinal products falling out of scope of Article 57(2) of Regulation (EC) No 726/2004 legal obligation
may be submitted on a voluntary basis in line with the requirements and business processes described
in this guidance (as applicable).
Note: EU IG V2.0 focuses primarily on the authorised products that fall under the scope of Article 57(2)
of Regulation (EC) No 726/2004. While investigational medicinal products will not be addressed in PMS
iteration 1, it is expected that EU IG V3 will further elaborate the specifications and business rules for
the Medicinal products falling out of scope of Article 57(2) of Regulation (EC) No 726/2004 as needed.
Submission of medicinal products authorised in EEA countries outside the
EU
In general, Iceland, Liechtenstein and Norway have, through the EEA agreement, adopted the
complete Union acquis on medicinal products and are consequently applying the EU rules governing
marketing authorisation procedures (i.e., national, centralised, decentralised and mutual recognition
procedures). However, the Commission’s decisions (including decisions granting marketing
authorisations) do not directly confer rights and obligations to holders of a marketing authorisation in
these countries. The marketing authorisations granted by the European Commission must be
transposed by the competent authorities of Iceland, Liechtenstein and Norway through corresponding
decisions based on relevant national laws. In such cases marketing authorisations granted in Iceland,
Liechtenstein and Norway are legally separate from the Commission’s decision when granting an MA.
Therefore, separate entries for the marketing authorisations granted in Iceland, Liechtenstein and
Norway should be submitted in PMS under Article 57(2) requirements of
Regulation (EC) No.
726/2004.
For medicinal products authorised in Liechtenstein, Norway and Iceland through the centralised
procedure the applicable country code (i.e., LI/NO/IS) shall be specified.
Submission of information on medicinal products with valid marketing
authorisation in the territory of Northern Ireland
Medicinal products authorised nationally in the UK (e.g., via the national, MRP or DCP procedure) with
valid marketing authorisation in the territory of Norther Ireland, and that are to be sold in Northern
Ireland under the Northern Ireland protocol, must be registered in PMS as they were in the XEVMPD.
Such products are in scope of Article 57(2) legal obligations.
• The country of authorisation of such medicinal product must reference ‘United Kingdom (Northern
Ireland) (XI)’ as the country of authorisation.
• EU authorisation procedure value must be referenced (e.g., national, decentralised, MRP etc.).
In accordance with the Protocol on Ireland/Northern Ireland, marketing authorisations granted via the
centralised procedure will continue to be valid in the territory of Northern Ireland. Therefore, no
separate AMP entry needs to be created for a medicinal product with a marketing authorisation granted
via the centralised procedure and valid in the territory of Northern Ireland.
Submission of medicinal products authorised under mutual recognition or
decentralised procedure in Liechtenstein
It is clarified in the Notice to Applicants (Volume 2A, Chapter 1) that on the basis of a bilateral
agreement between Liechtenstein and Austria automatic recognition of the Marketing Authorisations
granted in Mutual Recognition Procedure (MRP) or Decentralised Procedure (DCP) is operational. This

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 16/231
allows Liechtenstein to use Marketing Authorisations granted by Austria if the applicants have identified
Liechtenstein as CMS in the application form submitted with MRP or DCP applications. At the end of the
procedures, Austria grants authorisations that are recognised by Liechtenstein. This marketing
authorisation can be considered as a marketing authorisation granted in accordance with the
pharmaceutical acquis for the purpose of EU legislation.
Therefore, the marketing authorisation of these products must fulfil requirements provided for in, inter
alia, Regulation (EU) 726/2004 and Directive 2001/83/EC.
• The attachment to be used for reference in a medicinal product entity is an Austrian SmPC.
• The information shall however be provided in German.
Marketing authorisations granted by the Swiss authorities and recognised
by Liechtenstein
In the Notice to Applicants (Volume 2A, Chapter 1) it is also clarified that based on a bilateral
agreement between Liechtenstein and Switzerland, a Swiss marketing authorisation is effective in
Liechtenstein. This recognition has no effect outside the customs union between Switzerland and
Liechtenstein. Consequently, a marketing authorisation granted by the Swiss authorities and
recognised by Liechtenstein, while Switzerland does not apply the EU pharmaceutical acquis, cannot be
considered as a marketing authorisation granted in accordance with the pharmaceutical acquis for the
purpose of EU legislation and therefore falls outside the scope of, inter alia, Regulation (EU) 726/2004
and Directive 2001/83/EC.
Therefore, marketing authorisations granted by the Swiss authorities and recognised by Liechtenstein
fall out of scope of Article 57(2) requirements and do not therefore need to be submitted to PMS.
Submission of medicinal product data using FHIR
Medicinal product data shall be submitted to the Product Management System (PMS) using the FHIR
message format. The data elements for medicinal products presented in this guidance are based on the
following reference information:
• Summary of Product Characteristics (SmPC);
• Module 1.2 – Electronic Application form (eAF);
• relevant sections in Module 3 – Quality;
• medicinal product authorisation information (as referred to in the
Legal Notice on the
Implementation of Article 57(2) of Regulation (EC) No. 726/2004 published by the Agency);
• pharmacovigilance information (as referred to in the Legal Notice on the Implementation of Article
57(2) of Regulation (EC) No. 726/2004 published by the Agency).
Additional information on the process for submission can be found in EU IG Chapter 3 - Process for the
electronic submission of medicinal product information.
The contents of each document [i.e., Module 1.2 – Electronic Application form (eAF), relevant sections
in Module 3 – Quality, Summary of Product Characteristics (SmPC)] supporting the regulatory process
shall be aligned, where applicable, to ensure the discrepancies between the documents are minimized.
The content should enhance the quality of the product data reported in PMS. This requirement applies
to new medicinal products single entry in PMS.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 17/231
Based on the above principle, the SmPC as authorized/to be authorized is the main referring document
for data entry purposes.
However, for medicinal product entry already available in PMS (existing product data), following the
data load according to Chapter 7 - Migration guide, whenever the common contents of each of the
above supporting documentation are not aligned, the information available in the relevant sections in
Module 3 can be used to harmonize the values in PMS. This requirement applies provided data
confidentiality is ensured and if no additional complexity is added to the data entry in PMS. For
additional information, refer to section 1.3.1 of EU IG Chapter 8 – Practical example.
Note: Further information referring to existing product data will be made available in the EU IG
Chapter 9 - Process for submitting existing data on medicinal products authorised for human
use. This chapter is under development, and it will be made available at later stage.
Identifiers and defining characteristics of a medicinal product
entry in PMS
A medicinal product single entry in Product Management Service (PMS) is determined by the first
regulatory application to the relevant competent authority. This is further defined by a set of
characteristics that defines a medicinal product as a single unique entry in the PMS database.
Upon successful submission of product data to PMS, the system generates a set of unique identifiers:
• Product Management Service Identifier (PMS ID);
• Medicinal Product Identifier (MPID);
• Packaged Medicinal Product Identifier (PCID).
First submission means the first time the product data is introduced in PMS.
Following a successful submission of new medicinal product data, the applicable identifiers are
assigned to each PMS entity.
Each unique identifier is assigned based on a specific set of defining elements reported in the below
sections.
While only one PMS ID and MPID can be generated per medicinal product single entry at the time of
the first regulatory application, multiple PCIDs can be generated based on the number of authorized
packaged medicinal products.
Once the relevant unique identifiers are generated by the system, these can be used throughout the
medicinal product lifecycle, during the regulatory procedures (i.e., variation to the terms of the
marketing authorisations, renewal of the marketing authorisations etc.).
The medicinal product single entry is subject to versioning in PMS. The versioning is based on the
changes occurring during the medicinal product lifecycle. The subsequent versions may lead to the
assignment of new MPID and/or PCIDs.
Note: This version of the guidance does not report information on additional identifiers such as the
Pharmaceutical Product Identifier (PhPID). Further details on the related definitions and defining
elements will be available at later stage as it requires further discussions prior the implementation.
For further information related to the generation of the identifiers during the regulatory procedure,
refer to EU IG Chapter 3 - Process for the electronic submission of medicinal product information.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 18/231
Product Management Service Identifier (PMS ID)
In PMS, each individual medicinal product entry is assigned a single and unique PMS identifier (PMS
ID) that remains unchanged through the lifecycle of the medicinal product. The PMS ID is a
supplementary stable ID to any existing authorisation number or equivalent identifier as assigned by
an authorising body.
The PMS ID is automatically generated by the PMS system based on the first submission of the
authorised medicinal product data to PMS.
The PMS ID is only composed by digits. The value is maintained by a database sequence.
Example of PMS IDs would be: 00005005; 00001234; 00000567; 00000174.
Note: While the format of the identifier is confirmed, the number of digits may vary based on the
amount of medicinal product entries performed in PMS. These examples are illustrative only.
The defining characteristics for the creation of each single medicinal product entry in PMS (associated
to each PMS ID) includes:
• initial regulatory submission/application number;
• country (note: EU in the case of centrally authorised products);
• active substance
1
(or group of active substances contained in the same medicinal product);
• pharmaceutical form(s)
2
[*intended authorised pharmaceutical form(s)];
• medicinal product strength
3 4
(as intended for authorisation);
• Full (medicinal product) name as mentioned in Section 1: Name of the Medicinal Product of the
corresponding SmPC or other regulatory document and corresponding to the data element “Full
name” in Medicinal Product Name section;
• national identifier [marketing authorisation number(s)].
The following considerations apply to the concept of medicinal product entry in PMS and its associated
PMS ID:
• The defining elements are only used to generate the unique PMS ID at the time of the first
submission to PMS. Whenever one of the defining elements described above is different at the time
of the first submission of the relevant medicinal product to PMS, this constitutes a different product
entry in the PMS database and hence a different PMS ID is assigned.
• Once the PMS ID is assigned and linked to a medicinal product entry using the above-mentioned
defining characteristics, the PMS ID remains unchanged during the entire lifecycle of the
product.
1
A group of active substances contained in the same medicinal product includes fixed dose combinations or medicinal
products with more than one pharmaceutical product e.g., contraceptive pill and pessary containing different active
substances.
2 This definition applies to the authorised pharmaceutical form that may include one or more routes of administration in
certain term names available in the used RMS lists, e.g., concentrate and solvent for solution for injection/infusion; solution
for injection/infusion; emulsion for injection/infusion; solution for injection/infusion in pre-filled syringe. For the complete
list of RMS lists used refer to 1.5. (Authorised) pharmaceutical form.
3 Medicinal product strength may be expressed in different ways (e.g., strength per concentration / strength per unit of
presentation). In this scenario, the strength expressed as authorised should be taken as reference to determine the PMS
ID.
4 Includes products with more than one pharmaceutical product in the same medicinal product (e.g., starting packs with
different strengths)

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 19/231
• For nationally authorised products (NAPs), the PMS ID is aimed to be aligned with the concept of
individual medicinal products in regulatory application procedures (e.g., electronic application
forms).
• Whenever two medicinal products have the same attributes described above but are considered
two different regulatory procedures by the competent authority (e.g., duplicate medicinal products
due to duplicate regulatory applications submitted), these should be considered two different
medicinal products in PMS with two different PMS IDs and product lifecycles.
In addition, the ISO standard ISO11615 identifies a number of general conditions for both the
Medicinal Product Identifier (MPID) (see ISO11615, Section 8.2) and the Packaged Medicinal Product
Identifier (PCID) (see ISO11615, Section 8.3).
Medicinal Product Identifier (MPID)
5
A Medicinal Product Identifier (MPID) is a supplementary ID governed by the elements defined in ISO
standard ISO11615 and is assigned in addition to the PMS ID as well as any existing authorisation
number or equivalent identifier as assigned by the relevant competent authority. The MPID is
automatically generated by the PMS system following the successful submission of the medicinal
product data in PMS.
MPIDs are composed of the following elements:
• country code segment (ISO 3166-1 alpha-2 code elements);
• marketing authorisation holder (i.e., organisation ID) code segment;
• medicinal product code segment (i.e., a unique system generated medicinal product ID).
Any change of the initially submitted values related to these three code segments during the life cycle
of the medicinal product should result in the assignment of a new MPID generated by the system.
The country code segment of the MPID reflects the country where the medicinal product is
authorised and should be assigned in line with the ISO 3166-1 alpha-2 code elements.
• In case of a centralised product, the value “EU” is used for European Union.
• For Greece, the ISO code “GR” should be used instead of “EL” as the officially assigned country
code.
• For United Kingdom (Northern Ireland), the code “XI” should be used, as this is the standard that
the EU has decided upon to reference Northern Ireland.
The marketing authorisation holder code segment of the MPID is the MAH ID assigned by
Organisations Management Service (OMS)
following a successful submission of an organisation's
information in OMS. An organisation will be identified by the OMS LOC ID as this will be unique for an
organisation at its location.
Based on the LOC ID of the MAH the system will query OMS data to find the relevant ORG ID relevant
for the assignment of the MPID in the system. The ORG ID is therefore the defining element which is
part of the MPID structure.
If the required organisation and/or related location are not available, the addition of the unlisted
organisation and/or related location should be requested from OMS. Please refer to the process
described in the OMS Web User Manual available in the 'Documents' section of the Organisations
5
Concept of MPID - © CEN, reproduced with permission

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 20/231
Management Service (OMS) on how to submit and maintain organisation data in OMS. A unique
medicinal product ID code segment is the unique part of the MPID assigned to the medicinal
product according to the following defining attributes:
• Medicinal Product name: only the following name parts are considered for the generation of the
MPID: 1.14.3.3.1. , 1.14.3.3.2. Scientific name part, 1.14.3.3.10. Trademark or company name
part;
− The translations of the medicinal product name for medicinal products covered by the same
marketing authorisation (MA) number are considered to be the same in terms of medicinal
product name. Therefore, for countries such as Belgium and Luxembourg where there is more
than one official language, the names in French, Dutch and German are considered to be part
of the same medicinal product, hence are part of the same MPID and will not result in the
assignment of separate MPIDs. Refer to table 1b in section 1.18. Attached document.
− Likewise, the name of medicinal products authorised via the centralised procedure and covered
by the same MA number is also considered to be the same in terms of medicinal product name,
hence are part of the same MPID and will not result in the assignment of separate MPIDs.
Refer to table 1b in section 1.18. Attached document.
• the [authorised] pharmaceutical dose form(s) [refer to section 1.5. (Authorised) pharmaceutical
form];
• the active substance (s)/active moieties and their corresponding strength;
• device(s) where a medicinal product is combined with a medical device and where the
pharmacological, immunological or metabolic action is the principal mode of action; the medical
device is presented as part of the medicinal product.
• therapeutic indication(s) as authorised;
− Any changes to the listed therapeutic indications of the Medicinal Product as authorised may
result in the assignment of a new MPID.
− Where a change to the listed therapeutic indication(s) of the Medicinal Product as authorised is
introduced with the aim to modify an existing indication(s) (i.e., reword of the indication), a
new MPID will not be assigned.
− Where a change to the listed therapeutic indication(s) of the Medicinal Product as authorised is
introduced with the aim to extend or introduced in a completely new indication/target disease,
a new MPID will be assigned.
− Therapeutic indications will not be considered at the time of migration but only at a later point
in time.
• marketing authorisation number;
− This attribute is associated to the definition of MPIDs hence it may be a defining
characteristic of MPID, in accordance with the rules defined in the section ‘Marketing
authorisation information, therefore not all the changes to the Marketing Authorisation
number of the Medicinal Product as authorised may result in the assignment of a new MPID.
Example: The addition of a new pack without a change of the marketing authorisation number
at the medicinal product level will not result in the assignment of a new MPID. However, in this
instance a new PCID will be assigned. For further information refer to the examples in Annex I.
• legal status of supply (refer to section 1.7. Legal status of supply)

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 21/231
− The legal status of supply will not be considered at the time of migration but only at a later
point in time.
The unique medicinal product ID code segment is created with a successful data submission and
updated with a successful change to the above-mentioned attributes. The value of this segment is
meant to guarantee the uniqueness of the MPID in the system.
Example of MPIDs would be: EU-100000396-00180000 or IT-100030306-00000001.
Note 1: While the format of the identifier is confirmed, the number of digits may vary based on the
amount of medicinal product entries performed in PMS. These examples are illustrative only.
Changes to any of the elements listed above generated by data quality improvements should not
trigger the generation of a new MPID. Data quality assurance activities may involve corrections of
typographical errors, omissions or spelling errors which may not be linked to a regulatory submission.
For further information on how to manage the different types of changes in PMS, refer to section 3.
Maintenance submission of an authorised medicinal product (AMP) of EU IG Chapter 3 and section
Provenance in this Chapter.
Note 2: Some defining characteristics are not available at the time of migration and will be considered
only later. The MPID product ID code segment will be initially generated without considering these
values. At a later point in time, the same MPID product ID code segment will be assigned to the
extended set of characteristics. Therefore, in most cases, the MPID will not change when the additional
defining characteristic is added. For some records sharing the same MPID product ID code segment but
having different values for the newly added characteristic, a new MPID product ID code segment will
be generated. Thereafter, any change of this newly added characteristic will trigger a change of the
MPID product ID code segment according to the rules laid out above.
Packaged Medicinal Product Identifier (PCID)
6
For each Packaged Medicinal Product, a unique PCID shall be assigned by the system at the time of the
successful submission of the medicinal product data in PMS. This is supplementary to any
identifier/existing authorisation/approval number at package level assigned by the relevant competent
authority.
There are two components of a PCID:
• MPID for the Medicinal Product;
• package description code segment, which refers to a unique identifier for each package in the
context of the MPID e.g., 0001, 0002 etc.
Note: For authorisations which cover only one pack (authorisation number or equivalent identifier
is located at the level of the package medicinal product), one PCID will be assigned, with 0001 as
the package description code segment. For authorisations which cover more than one pack
(authorisation number or equivalent identifier is located at the level of medicinal product), a PCID
will be assigned to each pack. For further information on how to report the marketing authorisation
number(s), refer to sections 2.2. and 4.7.2. of this Chapter.
Example of PCIDs would be: EU-100000396-00020080-0001 or SE-100001745-00040001-0001.
6
Concept of PCID - © CEN, reproduced with permission

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 22/231
Note: While the format of the identifier is confirmed, the number of digits may vary based on the
amount of medicinal product entries performed in PMS. These examples are illustrative only.
Any change of the MPID component during the lifecycle of the medicinal product should result in the
update of the relevant PCID(s), generated by the system.
Package description code segment is assigned according to the following defining attributes:
• package item (container)(s) — the type, quantity (items per package), material(s);
• package component(s) — type, material(s);
• manufactured item(s) — manufactured dose form, unit of presentation, quantity (items per
package).
When the above defining attributes (MPID and/or attributes related to Package description code
segment) differ in any way a new PCID is assigned automatically by the system.
Relationship between PMS ID and ISO IDMP standard 11615 Medicinal
Product Identifier (MPID) and Packaged Medicinal Product Identifier
(PCID)
In accordance with the above sections, the following principles apply to the relationships between PMS
Medicinal Product identifier (PMS ID) and ISO standard ISO 11615 Medicinal Product Identifier (MPID)
and Packaged Medicinal Product Identifier (PCID):
• PMS ID remains stable from the first submission to PMS and throughout the lifecycle of the product
including all post-authorisation activities. PMS ID is linked to regulatory procedure numbers.
• MPIDs and PCIDs consist of a few defining elements (e.g., marketing authorisation holder for
MPIDs or package item container for PCIDs) that may change during the lifecycle of the product
resulting in an updated MPID or PCID as applicable linked to regulatory activities during the
lifecycle of the medicinal product. The result of changes to the MPID/PCID defining elements
occurring during the lifecycle of the product, superseded MPIDs/PCIDs become non-current.
• PMS ID, MPIDs and PCIDs share few defining elements (i.e., medicinal product name, authorised
pharmaceutical form). The above listed PMS ID defining elements support the assignment of the
relevant identifier based on the values entered only at the first submission of the authorised
medicinal product to PMS. Once generated the PMS ID remains stable during the entire lifecycle of
the medicinal product. If any change occurs to the initially submitted values of the shared PMS ID,
MPID and PCID defining elements, this will result only in the assignment of the updated MPIDs and
PCIDs, while the PMS ID will remain unchanged. PMS ID will be associated to the MPID concept but
will also be associated (within the PMS system) with all previous inactive MPIDs where MPIDs
changed during the lifecycle of the medicinal product.
• PMS ID has only one medicinal product associated which is identified by the MPID and is linked to
historical identifiers created during the medicinal product lifecycle. PMS ID may have one or
multiple packaged medicinal product (presentations) associated which are identified by the PCID.
This concept is further illustrated in the following example. Additionally, detailed fictional examples on
how IDs behave over the lifecycle of a medicinal product are presented as part of Annex I of this
document.
Example(s):

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 23/231
Imatinib Company A 25 mg tablets and Imatinib Company A 50 mg tablets are registered through
initial marketing authorisation application centralised procedure EMEA/H/C/000XXX/000.
Since this application includes one single pharmaceutical form and two strengths, two different unique
entries should be submitted for (one for the 25 mg tablets and then another for the 50 mg tablets)
then two unique PMS ID are set in accordance with the rules to set a unique PMS medicinal product.
Since this is the first submission, MPIDs and PCIDs are also set. MPIDs and PCIDs are subject to
change throughout the lifecycle of the medicinal product when any post-authorisation activities affect
any of their defining elements (e.g., MAH, legal status, package container).
The examples below provide a description on the evolution of the identifiers over the lifecycle of a
product. Additional examples are included in Annex I.
Note: Examples below are fictitious and for illustration purpose only.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 24/231
Imatinib Company A 25 mg tablets
Submission Procedure/application
number
PMS ID MPID Packs PCIDs
Initial MAA EMEA/H/C/000XXX/000 00005005 EU-100000396-
00000001
Blister
(alu) 1
tablet
Blister
(alu) 5
tablets
EU-100000396-
00000001-0001
EU-100000396-
00000001-0002
Addition of
new
manufacturer
EMEA/H/C/000XXX/IB/001 00005005 EU-100000396-
00000001
Blister
(alu) 1
tablet
Blister
(alu) 5
tablets
EU-100000396-
00000001-0001
EU-100000396-
0001-0002
Transfer of
MA
EMEA/H/C/000XXX/T/002 00005005
EU-100000497-
00000001
Blister
(alu) 1
tablet
Blister
(alu) 5
tablets
EU-100000497-
00000001-0001
EU-100000497-
0001-0002
Change of
primary
packaging
EMEA/H/C/000XXX/IB/003 00005005
EU-100000497-
00000001
Blister
(PVC) 1
tablet
Blister
(PVC 5
tablets
EU-100000497-
00000001-0003
EU-100000497-
00000001-0004
Change in
legal status
EMEA/H/C/000XXX/II/004 00005005
EU-100000497-
00000002
Blister
(PVC) 1
tablet
Blister
(PVC) 5
tablets
EU-100000497-
00000002-0003
EU-100000497-
00000002-0004

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 25/231
Imatinib company A 50 mg tablets
Submission Procedure/ap
plication
number
PMS ID MPID Packs PCIDs
Initial MAA
EMEA/H/C/000X
XX/000
00005006
EU-100000396-
00180000
Blister (alu) 1
tablet
Blister (alu) 5
tablets
EU-100000396-
00180000-0001
EU-100000396-
00180000-0002
Addition of new
manufacturer
EMEA/H/C/000X
XX/IB/001
00005006 EU-100000396-
00180000
Blister (alu) 1
tablet
Blister (alu) 5
tablets
EU-100000396-
00180000-0001
EU-100000396-
00180000-0002
Transfer of MA
EMEA/H/C/000X
XX/T/002
00005006
EU-100000497-
00180000
Blister (alu) 1
tablet
Blister (alu) 5
tablets
EU-100000497-
00180000-0001
EU-100000497-
00180000-0002
Change of
primary
packaging
EMEA/H/C/000X
XX/IB/003
00005006 EU-100000497-
00180000
Blister (PVC) 1
tablet
Blister (PVC) 5
tablets
EU-100000497-
00180000-0003
EU-100000497-
00180000-0004
Change in legal
status
EMEA/H/C/000X
XX/II/004
00005006 EU-100000497-
00180001
Blister (PVC) 1
tablet
Blister (PVC) 5
tablets
EU-100000497-
00180001-0003
EU-100000497-
00180001-0004

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 26/231
Access to identifiers
The unique identifiers listed in this guidance are generated by the PMS system upon successful
submission of the medicinal product single entry to Product Management Service (PMS).
Following the relevant submission of the medicinal product entry to PMS, the system will provide a
response which should allow the submitter to retrieve the medicinal product entry as is stored in PMS
including the generated unique identifiers.
For further information related to the generation of the identifiers during the process of product data
submission, refer to section 2. Initial submission of authorised medicinal product of EU IG Chapter 3 -
Process for the electronic submission of medicinal product information.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 27/231
User guidance
This section defines the attributes and provides business guidance and conventions for the electronic
submission of medicinal product data and document to be provided into PMS.
The medicinal product data to be completed in PMS are in the scope of Iteration 1 which is a sub-set
of the full data model defined in the ISO 11615 standard (Annex A).
The elements marked with [EXT] in Figure 1
below are extensions to the ISO model added in the
context of PMS Iteration 1 implementation.
To allow the reporting of multiple values some of the data elements available in this guidance are
repeatable fields. This information is reported in the table of the relevant data element(s). A clear
distinction on the possibility to repeat the entire class versus the different attributes of the class is also
reported in this chapter.
In line with the SPOR program, PMS is supported with referentials, organisation and substance master
data managed in RMS, OMS and SMS services respectively.
RMS, OMS and SMS terms are associated with unique identifiers assigned by the system at the time of
the creation of the master data. Further information is available on the SPOR Portal
.
Regarding the Referentials Management Service (RMS) operating model, RMS contains:
• Internally-managed lists (e.g., Marketing Authorisation Application Legal Basis, Special Precautions
for Storage). These lists are displayed with the record “EMA” under the “List Owner” column in the
RMS portal.
• lists owned by external maintenance organisations such as EDQM (pharmaceutical dose forms,
routes of administration, units of presentation, etc.); WHO (ATC Human, ATC Vet); MSSO
(MedDRA); ISO (Language). These lists are displayed with a record other than “EMA” under the
“List Owner” column in the RMS portal (i.e., EDQM, WHO CC, ISO, etc.)
Each RMS term is associated with a unique RMS identifier and can be mapped to other systems, when
applicable. Terms within RMS lists owned by external organisations (e.g., EDQM, WHO, MSSO) have an
RMS ID as well as the ID from the relevant source system. The latter is marked in RMS as the main
source for that term with the extended attribute “Is main source = Y” to ensure that the ID from the
external list owner is displayed as the source ID (e.g., EDQM ID, ATC code from WHO, MedDRA code,
etc.) and that this ID is displayed in the “Source ID” column in the RMS web portal.
As a general principle, PMS consume referentials from the RMS lists, hence the relevant RMS identifiers
referring to the applicable RMS term are required to be submitted into PMS.
The user shall select the most suitable RMS term to reflect the structuring of the product data by
applying standardized master data. The user should consider that not every available RMS term even if
technically selectable to PMS is applicable from a regulatory point of view.
There are cases where the RMS terms contain the RMS ID which are mapped to the identifier of the
external source (i.e., the source ID). When submitting product information into PMS, the RMS ID is the
identifier to be used (therefore the source ID, if existing, should not be used). This principle applies to
both internally-managed lists and externally-managed lists.
However, there are two externally-managed lists where the above reported principle does not apply.
These exceptions are:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 28/231
• Medical Dictionary For Regulatory Activities – MedDRA (RMS ID 100000000006). managed
by MSSO.
• Anatomical Therapeutic Chemical classification system – Human (RMS ID 100000093533),
managed by WHO CC.
In these two cases, the external ID from the relevant source system (source ID) can also be used to
submit the relevant product information into PMS, as an alternative to using the RMS term IDs. Upon
selection of the external source ID, the PMS system will automatically retrieve and show the
appropriate RMS term as result of the mapping mechanisms explained above. In this case, the version
of the external identifier shall correspond to the latest updated version available in RMS.
References to referentials, organisation and substance data either through FHIR Identifiers or
CodeableConcept data types shall specify a “system” and “value” pair as per FHIR specification of
these data types. For referentials and substance data a FHIR extension will be used to specify the
version number of the data. The current document offers guidance on the “value”. The “system” is a
constant which differs per type of data and is per convention the SPOR API URL of the resource
referred to. Details on how to form the FHIR data for these types of references can be found in Chapter
VI – SPOR API Technical Specification.
The description of the requirements for each set of information and each data element is presented in
the following tabular format:
Tag Description
User Guidance
The definition of the data element, the convention and the condition under
which the information should be provided in the context of medicinal
product data for human use into PMS. This applies in the context of the
regulatory submission (initial submission and maintenance of the product
information) as well as notification, data enrichment and nullification of
product data.
Repeatable The cardinality of the data elements specifying whether multiple values for
the information can be applied. A class could be repeatable but with
individual data fields repeatable or not. The complete set of the data fields
is repeated in case the class is repeatable.
Conformance Whether the information should be provided on mandatory, conditional or
optional basis. A class could be conditional and data fields belonging to the
class could be mandatory. Once the conditions for the class are fulfilled, all
mandatory data fields shall be fulfilled. If the conditions are not fulfilled,
none of the data fields belonging to the class shall be provided.
• Mandatory: the provision of the product data is compulsory; therefore,
the field(s) shall be populated with the available information.
• Conditional: the provision of the product data is compulsory only if the
information is available. Therefore, the field(s) shall be populated
accordingly.
• Optional: the provision of the product data is not mandatory; however,
the field(s) can be populated if the information is available.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 29/231
Tag Description
Data Type The type of data that should be specified as defined in the FHIR message
(e.g., CodeableConcept refers to use of controlled terminologies; string
refers to free text data; numeric values).
Value The values applicable to the data element (e.g., reference to the SMS,
OMS or relevant RMS list).
ISO Element Name Any mapping to ISO IDMP standards, when applicable.
ISO Path The mapping of the ISO IDMP technical specifications, when applicable.
FHIR Element Name The name of the FHIR element as presented in the FHIR resource list.
FHIR Path The FHIR data model path as presented in the FHIR resource list.
FHIR Complementary
Information
Additional information on expected values for FHIR data elements
expected in addition to the value provided, for example the system to use
for an identifier or which type of date is specified.
Additional information on the technical aspects such us reference systems, cardinality, data types,
extensions, examples, etc. refer to Chapter VI – SPOR API Technical Specification and the latest
version of the HL7 FHIR Specification is accessible through this link.
Note: This current version 2.1 of the Implementation Guide is based on HL7 FHIR Specification version
4.4.0 (FHIR R5 Preview 3) while the created FHIR messages examples are based either on 4.2.0 (FHIR
R5 Preview 2) (source: http://hl7.org/fhir/2020Feb/
) or 4.4.0 (FHIR R5 Preview 3) (source:
http://hl7.org/fhir/2020May/).The HL7 FHIR Specification version mentioned in this guidance is a
preview version, therefore it is not expected to be the version used at the time of the go-live of PMS.
The go-live is intended to implement a major version of the FHIR specification such as FHIR R5. The
guidance will be updated in due course to reflect the latest version used at the time of the go-live of
PMS.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 30/231
Figure 1: Iteration 1 ISO IDMP information model for authorised medicinal products with PMS
extensions.
7
8
Note that not all the data elements showed in Figure 1 are implemented in the PMS data model.
Therefore, these are not listed in the current version of the EU IG Chapter 2.
7
In Figure 1 not all the data fields shown are part of the Iteration 1
8
Figure of data model - © CEN, reproduced with permission

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 31/231
Provenance
The full information concerning the general overview of the submission of medicinal product data shall
be specified in Provenance.
This data element is used to track information about the activity performed in PMS in the context of a
creation or update of a version of a medicinal product dataset submission message, describing the
target and agent involved. This information is used to convey a description of the action performed by
specifying what the action is, why it is occurring and by who it is performed.
Provenance resources are prepared by the application that initiates the create/update etc. of the
resource.
Figure 2 : FHIR Resource Provenance (source : http://www.hl7.org/fhir/
)
The Provenance class and the individual attributes of this class are mandatory and not repeatable.
Provenance Class Description
Repeatable
No
Conformance
Mandatory
Provenance class contain the flowing data elements.
Reason
Tag Description
User Guidance The reason for the submission of the medicinal product dataset in PMS
shall be specified.
The applicable value from the RMS list shall be selected to indicate the
context in which the creation or change of/to the medicinal product data
occurs.
The dataset submission should be marked as:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 32/231
Tag Description
• Regulatory Submission - Initial: this is applicable to data submission
linked to initial regulatory procedures/applications submissions such as
initial Marketing Application or Line Extension;
• Regulatory Submission – Maintenance: this is applicable to report any
change to the terms of the marketing authorisation requiring
regulatory assessment (e.g., variations, renewals, transfer of
marketing authorisation).
• Notification: applicable if any change to the terms of the marketing
authorization without Regulatory assessment is submitted. This term is
to be used for data submission not linked to regulatory
procedures/applications. The following situations apply:
− Changes of (Pharmacovigilance) master file – Section 1.15
− Changes of QPPV Contact (QPPV) – Section 1.16
− Changes on contact information for pharmacovigilance enquiries –
Section 1.17
− Change to the Authorisation Status changes triggered by
Competent Authorities – Section 2.4
− Changes to the marketing status – Section 4.6
− Changes to the Data Carrier Identifier – Section 4.8.6.
• Enrichment - Full: This reason should be used when the full FHIR
dataset is provided to complete the full record following IDMP rules
and compliance.
• Enrichment – Partial: This reason should be used when specific
resources are provided to partially enrich migrated data or correct
erroneous migrated data.
• Nullification: used to flag as "nullified" medicinal product entities
created by mistake or entities provided erroneously.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL The applicable value as listed from the RMS list [list will be created in
v2.2]
Value(s)
As listed in the RMS list. This list is to be created.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Reason
FHIR Path
Provenance.reason
For further information related to the process of submission, refer to sections 2 and 3 of EU IG Chapter
3 - Process for the electronic submission of medicinal product information.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 33/231
Note: Further RMS terms may be created in due course if additional path related to the reason of
submission are deemed necessary.
Target
Tag Description
User Guidance The reference to the Medicinal Product PMS ID which is impacted by the
activity shall be specified.
Repeatable
No
Conformance
Mandatory
Data Type
Reference
RMS URI/URL
Not applicable
Value(s)
Reference to Medicinal Product.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Target
FHIR Path
Provenance.target
Recorded
Tag Description
User Guidance Timestamp of when the activity took place shall be specified.
Repeatable
No
Conformance
Mandatory
Data Type
Timestamp
RMS URI/URL
Not applicable
Value(s) The timestamp format is YYYY-MM-DDThh:mm:ss.sss+zz:zz (e.g., 2020-
02-07T14:53:33.134+02:00 or 2021-01-01T00:00:00Z). The time should
be specified at least to the second and should include a time zone.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Recorded
FHIR Path
Provenance.recorded
Sender
Tag Description
User Guidance The Organisation identifier of the entity performing the activity shall be
specified using the location identifier (LOC ID) linked to the organization as
listed in the Organisation Management System (OMS) following a
successful registration of the organisation’s details.
If the required organisation and/or related location are not available, the
addition of the unlisted organisation and/or related location should be
requested from OMS using the process described in the OMS Web User
Manual in the 'Documents' section of the Organisation Management
System (OMS).

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 34/231
Tag Description
When the LOC ID is specified, the system would give the information to
which ORG ID the selected location is linked in the back end. This
information will be shown through the PMS User Interface.
Repeatable
No
Conformance
Mandatory
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
As listed in SPOR OMS service (LOC ID)
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Who
FHIR Path Provenance.agent.who
1. Medicinal product
The full information on Medicinal Product as presented in the FHIR Resource MedicinalProductDefinition
is presented in the figure 3
below. In the context of the Iteration 1 of the PMS implementation, only
the information highlighted in red is in scope and should be provided according to the rules and
guidance as described in this section.
Figure 3: FHIR Resource MedicinalProductDefinition (source: http://www.hl7.org/fhir/)
The Medicinal Product Definition class and its attributes is mandatory and not repeatable. The
individual attributes of this class shall be populated as applicable.
Medicinal Product Definition Class Description
Repeatable
No
Conformance
Mandatory

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 35/231
1.1. Product Management Service Identifier (PMS ID)
PMS ID will be assigned to each medicinal product single entry following the first submission of the
authorised medicinal product data to PMS. It is a unique, stable and permanent ID supplementary to
any existing authorisation number or equivalent identifier as assigned by an authorising body.
The PMS ID is composed only by digits.
This attribute is automatically generated by the PMS system and remains unchanged through the
lifecycle of the medicinal product.
The PMS ID shall be specified when performing any maintenance related activity of the medicinal
product data.
Tag Description
User Guidance The PMS ID will be assigned following a successful initial submission of
the medicinal product information in PMS.
Repeatable
No
Conformance • For first data submission: Not applicable – ID generated by the system
•
For data maintenance: Mandatory
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
ID generated by the system
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Identifier
FHIR Path
MedicinalProductDefinition.id
1.2. Medicinal Product Identifier (MPID)
9
MPID shall be assigned to each authorised medicinal product; it is a supplementary ID to any existing
authorisation number or equivalent identifier as assigned by an authorising body in a region.
The MPID is defined by the following segments:
• country code segment (ISO 3166-1 alpha-2 code elements);
• marketing authorisation holder (i.e., organisation ID) code segment;
• medicinal product code segment (i.e., unique medicinal product ID).
Any change of the values related to these three code segments (as described in the Introductory
section) should result in the assignment of a new MPID.
This attribute is automatically generated and maintained by the PMS system.
Tag Description
User Guidance The MPID will be assigned following a successful first submission of the
medicinal product information in PMS.
Repeatable
No
Conformance For first data submission and data maintenance: Not applicable – ID
generated by the system
9
Concept of MPID - © CEN, reproduced with permission

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 36/231
Tag Description
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
ID generated by the system
ISO Element Name
MPID
ISO Path
/MedicinalProduct/MPID
FHIR Element Name
Identifier
FHIR Path
MedicinalProductDefinition.identifier
FHIR Complementary
Information
MedicinalProductDefinition.identifier.system value is
“http://ema.europa.eu/fhir/mpId”

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 37/231
1.3. Domain
Domain describes whether the type of medicinal product is for human or for veterinary use.
Tag Description
User Guidance The domain shall be provided as a term ID.
• The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
In the context of the implementation of PMS iteration 1 only medicinal
product for human use shall be provided and therefore the value “human
use” applies.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000004
Value(s)
Listed in the Domain RMS List
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Domain
FHIR Path
MedicinalProductDefinition.domain
Example(s):
Human use (100000000012)
1.4. Type
ISO IDMP distinguishes between Authorised Medicinal Product and Investigational Medicinal Product.
In the context of the implementation of PMS iteration 1, only Authorised Medicinal product is
supported.
“Authorised Medicinal Product” covers Medicinal products for which a marketing authorisation in
EU/EEA was requested or granted, including those for which the marketing authorisation is no longer
valid. The product itself does not need to be authorised/approved and this information should be
reflected in 2.4 Authorisation status (medicinal product level) and in 4.6.4. Authorisation status
(package level), when applicable.
“Authorised Medicinal Product” also covers:
• traditional use registration for herbal medicinal products (Article 16a of Directive 2001/83/EC);
• simplified registration for homeopathic medicinal products (Article 14 of Directive 2001/83/EC);
• medicinal products within the scope of Article 5 of Directive 2001/83/EC i.e., 'Named patient use'
falling under Article 5(1) and 'EU Distribution Procedure' under Article 5(2);
• parallel distribution/parallel import of medicinal products (Article 76(3) and (4) of Directive
2001/83/EC);
• medicinal products authorised outside the European Economic Area (EEA) or following a non-EU
procedure;

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 38/231
• extemporaneous Medicinal products (e.g., medicinal products prepared in a pharmacy based on a
medical prescription such as pharmacy preparations);
• intermediate products intended for subsequent processing by an authorised manufacturer.
Investigational medicinal products as defined in the Directive 2001/20/EC Article 2(d) are not covered
in PMS iteration 1.
Tag Description
User Guidance The type shall be provided as a term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
In the context of the implementation of PMS iteration 1, only Authorised
Medicinal product shall be provided.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000025915
Value(s)
As listed in the Medicinal Product Type RMS list.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Type
FHIR Path
MedicinalProductDefinition.type
Example(s):
Authorised Medicinal Product (200000025916)
Investigational Medicinal Product (200000025917)
1.5. (Authorised) pharmaceutical form
The pharmaceutical form as submitted for authorisations or as authorised by regulatory authorities and
as reflected in regulatory documents shall be provided.
Pharmaceutical form might be authorised or submitted for authorisation as follows:
• Combined pharmaceutical form: when two or more manufactured dose forms that are intended to
be combined to create a single administrable dose form. In this case the RMS list “Combined
pharmaceutical form” shall be used.
Example: powder and solvent for solution for injection
• Pharmaceutical dose form: when the authorised dose form involves a single standard term dose
form which does not fall into one of the other categories and which may or may not undergo
transformation prior to administration to the patient. In this case the RMS list “Pharmaceutical
dose form” shall be used.
Example: tablet, capsule, solution for injection
• Combined terms: in special cases (e.g., identical products which may be distinguished only by
reference to the container), the information about the immediate container can be included in the
authorised pharmaceutical form. In this case the RMS list “Combined term” shall be used.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 39/231
Example: solution for injection in pre-filled syringe
• Combination Package: medicinal products may consist of two pharmaceutical products that
correspond with two different administrable dose forms (e.g., hard capsule and cream) that form
individual entities which do not need combining for administering to the patient. In this case the
RMS list “Combination Package” shall be used.
Example: Cream + vaginal tablet
Tag Description
User Guidance The authorised pharmaceutical dose form(s) or the dose form submit shall
be provided as an RMS term ID.
Repeatable
Yes
Conformance
Mandatory
Data Type
Codable Concept
RMS URI/URLs • https://spor.ema.europa.eu/v1/lists/200000000006
• https://spor.ema.europa.eu/v1/lists/200000000004
• https://spor.ema.europa.eu/v1/lists/200000000007
•
https://spor.ema.europa.eu/v1/lists/200000000008
Value(s) As applicable in one of the SPOR RMS lists:
• Combined pharmaceutical dose form
• Pharmaceutical dose form
• Combined term
•
Combination Package
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
authorisedDoseForm
FHIR Path MedicinalProductDefinition.extension.authorisedDoseForm
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
1.6. Combined pharmaceutical dose form
'Combined pharmaceutical dose form' is a single term to describe two or more manufactured dose
forms that are intended to be combined to create a single administrable dose form. If there is no
combining needed in order to prepare the administrable dose form (e.g., medicinal product is
composed of a single manufactured item that equals the administrable dose form) then the 'Combined
pharmaceutical dose form' is left blank.
Medicinal products may consist of two pharmaceutical products that correspond with two different
administrable dose forms (e.g., hard capsule and cream) that form individual entities which do not
need combining for administering to the patient. In these cases, the field should be left blank.
To illustrate this concept, additional examples are reflected below:
Example(s):
• 'Powder' and 'solvent' are two manufactured items that shall be combined to create a single
pharmaceutical product. The administrable dose form that will be created using these two
manufactured items and administered to the patient will be 'solution for injection'. The combined

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 40/231
pharmaceutical dose form to be used in this case is the standard term ‘Powder and solvent for
solution for injection’.
• “Film-coated tablet” involves a single manufactured item. This manufactured item corresponds with
the administrable dose form (no previous preparation/combination with other manufactured item is
needed). Therefore, this field should be left blank.
• “Oral Capsule” & “External Cream” correspond with two different administrable dose forms which
do not need combining for administering to the patient. Therefore, this field should be left blank.
Tag Description
User Guidance The combined pharmaceutical dose form(s) shall be provided as an RMS
term ID.
Repeatable
No
Conformance
Conditional
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000000006
Value(s)
As applicable in SPOR RMS Combined pharmaceutical dose form list
ISO Element Name
Combined Pharmaceutical Dose Form
ISO Path
/MedicinalProduct/CombinedPharmaceuticalDoseForm
FHIR Element Name
combinedPharmaceuticalDoseForm
FHIR Path
MedicinalProductDefinition.combinedPharmaceuticalDoseForm
1.7. Legal status of supply
Legal status of supply of the medicinal product as authorised by the relevant competent authority shall
be specified.
In the scenario that legal status of supply differs at package level (different legal status for different
package sizes of the same medicinal product), this information at medicinal product level is to be
populated with the RMS term “Medicinal product subject to medical prescription exempt for some pack
sizes”. For those cases, the legal status of supply shall be entered at package level only refer to section
4.5. - Legal Status of Supply at Package Medicinal Product Level. In these cases, the field on product
level will show the information that the “Medicinal product subject to medical prescription exempt for
some pack sizes”.
Tag Description
User Guidance The legal status of the medicinal product's supply, as authorised by the
competent authority and applicable in the region, shall be specified using a
term ID.
• The applicable value shall be selected from the term ID as available in
the applicable Referentials Management Service (RMS) list.
• For Centralised Authorised Products (CAPs), this information is
retrieved from Annex II.B - CONDITIONS OR RESTRICTIONS
REGARDING SUPPLY AND USE and from section 4.2 – Posology of the
Product information.
• For Nationally Authorised Products (NAPs), this information may be
retrieved from different sources that includes from the Product
information the Summary of Product Characteristics (SmPC), Package

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 41/231
Tag Description
Leaflet (PL) or other annexes) to National Register of Medicinal
Products.
• The legal status for the supply is usually defined at medicinal product
level and should be specified as Medicinal product subject to medical
prescription or Medicinal product not subject to medical prescription;
• In the scenario that legal status for the supply is defined at package
level only (different legal status for different package sizes of the same
medicinal product), the term Medicinal product subject to medical
prescription exempt for some pack sizes can be specified. For those
cases, the legal status for the supply must be entered at package level
(see section 4.5. - Legal Status for the Supply at Package Level).
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072051
Value(s)
As listed in the Legal Status for the Supply RMS list.
ISO Element Name Legal Status of Supply
ISO Path
/MedicinalProduct/MarketingAuthorisation/LegalStatusOfSupply
FHIR Element Name
legalStatusOfSupply
FHIR Path
MedicinalProductDefinition.legalStatusOfSupply
Example(s):
Medicinal product subject to medical prescription (100000072084)
Medicinal product not subject to medical prescription (100000072076)
Medicinal product subject to medical prescription exempt for some pack sizes(example of term to be
created)
1.8. Additional monitoring indicator
Indication whether the medicinal product is subject to additional monitoring (black triangle/symbol) in
accordance with Art. 23 of Regulation (EC) No 726/2004.
Tag Description
User Guidance The value indicating whether the medicinal product is subject to additional
monitoring should be specified if the medicinal product is subject to
additional monitoring.
Repeatable
No
Conformance
Mandatory
Data Type
Boolean

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 42/231
Tag Description
RMS URI/URL
Not applicable
Value(s)
True / False
ISO Element Name
Additional Monitoring Indicator
ISO Path
/MedicinalProduct/AdditionalMonitoringIndicator
FHIR Element Name
additionalMonitoringIndicator
FHIR Path
MedicinalProductDefinition.additionalMonitoringIndicator
Example(s):
In this case as the medicinal product is subject to additional monitoring, the Boolean concept “True”
shall be selected.
1.9. Orphan designation
Orphan designation is a status assigned to a medicine intended to treat a rare condition as defined in
Regulation (EC) No 141/2000.
Medicinal products designated as Orphan Medicinal Products are listed in the Register of designated
Orphan Medicinal Products published by the European Commission accessible through the following
link.
This section shall be completed when an authorised medicinal product is designated as an orphan
medicinal product bearing one or more orphan indications. If the authorised medicinal product is not
designated as an orphan medicinal product this section shall be left blank.
The authorised medicinal product can be associated to more than one orphan drug designation (ODD).
Example(s):
Medicinal product with orphan designation
In FHIR resources, Orphan Designation is captured as a special type of RegulatedAuthorization (refer
to Figure 4
), which illustrates how the resource is used in the context of an Orphan Designation:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 43/231
Figure 4: Orphan Designation Status as captured by the RegulatedAuthorization class in FHIR (Source:
http://www.hl7.org/fhir/)
The Orphan Designation class is conditional and repeatable while the individual attributes of this class
are not repeatable and shall be populated as applicable.
Orphan Designation Class Description
Repeatable
Yes
Conformance
Conditional
1.9.1. Regulatory authorisation type
Regulatory applications may be submitted to obtain different type of authorisations or regulatory
entitlements such as orphan designations, Advanced therapy medicinal product (ATMP) classification,
marketing authorisations etc. in accordance with the current European regulatory framework for
medicinal products. The regulatory authorisation type “Orphan Designation” shall be specified in this
section.
Tag Description
User Guidance The type of regulatory authorisation shall be specified, when applicable.
Please note that the RegulatedAuthorization type shall be set as Orphan
Designation.
The applicable value shall be selected from the term ID as available in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/220000000060
Value(s) • As listed in the Regulatory Entitlement Type RMS list.
•
The value “Orphan Designation” shall be selected.
ISO Element Name
Not Applicable
ISO Path
Not Applicable
FHIR Element Name
Type
FHIR Path
RegulatedAuthorization.type

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 44/231
1.9.2. Orphan designation status
Tag Description
User Guidance The following Orphan designation regulatory status shall be specified
where applicable:
• Valid: starting from the date of the EC decision of the initial Marketing
Authorisation Application OR variation, granting an indication covered
by an orphan designation.
• Expired: when the ME period for that orphan designation has expired,
at the end of the 6, 10 or 12-year period of ME.
• Withdrawn: when the Orphan Designation of the medicinal product is
withdrawn by the MAH (via a specific procedure).
• The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
The Orphan Designation status of the medicinal product (where applicable)
can be found in the individual product entry at the EC Register of medicinal
product accessible via this link.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072049
Value(s)
As listed in the Regulatory Entitlement Status RMS list.
ISO Element Name
Orphan Designation Status
ISO Path
/MedicinalProduct/OrphanDesignationStatus
FHIR Element Name
status
FHIR Path
RegulatedAuthorization.status
Example(s):
Valid, Expired, Withdrawn
1.9.3. Orphan designation number
Tag Description
User Guidance The Orphan designation number granted by the European Commission as
listed in the Register of designated Orphan Medicinal Products shall be
specified.
This is a repeatable field.
Repeatable
No
Conformance
Mandatory
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s) As assigned by the European Commission decision in Orphan Designation
register.
ISO Element Name
OrphanDesignationNumber
ISO Path
/MedicinalProduct/OrphanDesignation/OrphanDesignationNumber
FHIR Element Name
Identifier

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 45/231
Tag Description
FHIR Path
RegulatedAuthorization.identifier
FHIR Complementary
Information
RegulatedAuthorization.identifier.system value is
“http://ema.europa.eu/fhir/marketingAuthorizationNumber”
Example(s):
EU/3/XXXX/53
1.9.4. Orphan designation status date
Tag Description
User Guidance The date when the orphan designation status of the medicinal product was
assigned shall be specified where applicable. The following dates are
applicable per status:
• Valid: date of the EC decision of the initial Marketing Authorisation
Application that designates the medicinal product as orphan.
• Expired: date of expiration of the market exclusivity period for the
medicinal product.
• Withdrawn: date of withdrawal of the Orphan Designation of the
medicinal product by the MAH.
The date when the orphan designation status of the medicinal product was
assigned can be found in the individual product entry in the EC Register of
medicinal product accessible via this link.
Repeatable
No
Conformance
Mandatory
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s) A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
OrphanDesignationAuthorisationDate
ISO Path /MedicinalProduct/OrphanDesignation/OrphanDesignationAuthorisationDat
e
FHIR Element Name
statusDate
FHIR Path
RegulatedAuthorization.statusDate
Example(s):
2017-10-23
1.9.5. Market exclusivity start date
Tag Description
User Guidance The date when the market exclusivity of the orphan medicinal product
starts shall be specified where applicable and shall reflect the date of the
authorisation of the first indication. The date of start of the market

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 46/231
Tag Description
exclusivity of the orphan medicinal product can be found in the individual
product entry at the EC Register of medicinal product accessible via this
link.
Repeatable
No
Conformance Mandatory
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s) A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
validityPeriod
FHIR Path
RegulatedAuthorization.validityPeriod.start
Example(s):
2015-09-01
1.10. Paediatric use indicator
The value indicating whether the medicinal product is authorised and explicitly indicated for paediatric
use shall be specified.
As per Regulation (EC) No 1901/2006:
• Article 1 - ‘paediatric population’ means that part of the population aged between birth and 18
years;
• Article 3 - ‘medicinal product authorised for a paediatric indication’ means a medicinal product
which is authorised for use in part or all of the paediatric population and in respect of which the
details of the authorised indication are specified in the summary of the product characteristics
drawn up in accordance with Article 11 of Directive 2001/83/EC.
The above-mentioned definitions can be used with the authorised indications of a medicinal product
when completing this section. This information is to be inferred from section 4.1-Therapeutic
indications of the SmPC. In certain cases, the target adult/paediatric population is not referred in
section 4.1 of the SmPC; this information can also be inferred from section 4.2 – Posology and method
of administration of the SmPC.
Example(s):
Example 1: information of paediatric use included in section 4.1 of the SmPC

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 47/231
Section 4.1 Therapeutic indications of the SmPC states: Levetiracetam ProductXYZ is indicated as
monotherapy in the treatment of partial onset seizures in adults and adolescents from 16 years of age
with newly diagnosed epilepsy.
Example 2: information of paediatric use not reflected in section 4.1 of the SmPC but included in
section 4.2 of the SmPC
Section 4.2 Posology and method of administration of the SmPC states:
Posology
Monotherapy for adults and adolescents from 16 years of age.
Therefore, in both cases, the value ‘Yes' shall be specified.
Tag Description
User Guidance Paediatric use indicator shall be completed to indicate whether a medicinal
product is authorised for a paediatric indication with the following values:
• Yes: shall be selected if an indication for paediatric population (children
under the age of 18) is stated in Section 4.1 Therapeutic indications of
the SmPC and/or a posology is stated for any subset of the paediatric
population in Section 4.2. Posology and method of administration of
the SmPC.
• No: shall be selected if the medicinal product does not meet any of the
above condition.
Repeatable
No
Conformance
Mandatory
Data Type
Boolean
RMS URI/URL
Not applicable
Value(s)
True / False
ISO Element Name
Paediatric Use Indicator
ISO Path
/MedicinalProduct/PaediatricUseIndicator
FHIR Element Name
paediatricUseIndicator
FHIR Path
MedicinalProductDefinition.paediatricUseIndicator
1.11. Full indication text
The description of the authorised full therapeutic indication(s) shall be described in text as reflected in
Section 4.1 Therapeutic Indications of the corresponding SmPC or another regulatory document (e.g.,
Package leaflet) if no SmPC is available.
• centrally authorised products (CAPs): only the English version of the full indication text is
mandatory to be submitted;
• for nationally authorised products (NAPs) including products registered through the mutual
recognition procedure (MRP), decentralised procedure(DCP) and national procedure (NP):
− the text shall be provided in the national language, as authorised by the competent authority
and as stated in section 4.1 Therapeutic Indications of the corresponding SmPC. Provision of
additional translation in English is on optional basis,
− in countries with multiple languages (e.g., Belgium) the full therapeutic indication of at least
one local language is mandatory,

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 48/231
Additional examples can be found below.
Tag Description
User Guidance The description of the authorised full therapeutic indication(s) shall be
described in text as reflected in Section 4.1 Therapeutic Indications of the
corresponding SmPC or other regulatory document (identical copy and
paste).
This is a repeatable field where full indication text is included in more than
a single language.
Only in cases where rich text shall be provided, Markdown language can be
used, and the content of this field shall adhere to Markdown format.
Repeatable
Yes
Conformance Mandatory (at least one language)
Optional (for additional languages in countries with multiple languages)
Data Type
Markdown
RMS URI/URL
Not applicable
Value(s)
Free text or markdown text for rich content
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Indication
FHIR Path
MedicinalProductDefinition.indication
FHIR Complementary
Information
The data element is repeatable by using a FHIR extension. Please refer to
Chapter VI – SPOR API Technical Specification for the details of the
extension URL.
Example(s):
Indication text: Adults, elderly and Children over 12 years: Rheumatic or muscular pain,
backache, neuralgia, migraine, headache, dental pain, dysmenorrhoea,
feverishness, symptoms of colds and influenza.
Indication text: Medicinal Product XX esta indicado en niños a partir de 3 meses y hasta 12
anos en: Alivio sintomático de los dolores ocasionales leves o moderados.
Estados febriles.
Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 49/231
1.11.1. Language
This section described how to populate information related to the language of the indication. The
provision of the language is mandatory.
Tag Description
User Guidance The language of the medicinal product indication, as approved by the
regulatory authority and indicated in the corresponding regulatory
document(s) shall be specified as a term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072057
Value(s)
As indicated in the Language RMS list
ISO Element Name
Not Applicable
ISO Path
Not Applicable
FHIR Element Name
valueCode
FHIR Path MedicinalProductDefinition.indication.extension.language
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
1.12. EURD ID
The legal requirements for submission of Periodic Safety Update Reports (PSURs) are established in
Regulation (EU) No 1235/2010, Directive 2010/84/EU and in Commission Implementing Regulation
(EU) No 520/2012.
The 2010 legislation introduced the principle of EU single assessment where a substance is authorised
in more than one Member State. The aim is to harmonise and strengthen the safety and benefit-risk
review of medicines across the European Economic Area.
The Agency maintains a list of EU reference dates and frequency of submission of PSURs
(EURD list)
for active substances contained in medicines in the EU. This list is updated on an ongoing basis.
MAHs are required to submit PSURs to national competent authorities and the Agency according to the
dates published in this list.
Tag Description
User Guidance When the medicinal product is part of a PSUR single assessment procedure
in accordance with the European Union reference date dates and frequency
of submission of periodic safety reports list (EURD list), the EURD ID shall
be provided. The EURD ID shall be derived from the procedure number of
the PSUR single assessment, which is published in the EURD list Excel file
available on the 'Periodic safety update reports' webpage.
In case of medicinal product with no active ingredient, the EURD ID can be
left blank.
Repeatable
No
Conformance
Conditional

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 50/231
Tag Description
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s) The EURD ID should be included without any leading zeros and as listed in
the Excel file “List of European Union reference dates and frequency of
submission of periodic safety update reports”
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Identifier
FHIR Path
MedicinalProductDefinition.identifier
FHIR Complementary
Information
MedicinalProductDefinition.identifier.system value is
“http://ema.europa.eu/fhir/eurdId”
Example(s):
For procedure number PSUSA/00000009/202103, the EURD ID is 9. This value will be used for all
medicinal products containing ‘5-aminolevulinic acid’.
1.13. Product classification
The medicinal product can be classified according to various classification systems. In the context of
PMS iteration 1, the product classification describes the following product information:
• XEVMPD Medicinal product types;
• Legal basis;
• ATC Code(s);
• Medicinal product category
• Genetically modified organisms
The Product Classification class is mandatory and not repeatable while the individual attributes of this
class are repeatable and shall be populated as applicable.
Product Classification Class Description
Repeatable
No

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 51/231
Product Classification Class Description
Conformance
Mandatory
1.13.1. xEVMPD product type information
The type of medicinal products shall be completed. Product type is for the purpose of
pharmacovigilance fees in accordance with the current XEVMPD controlled list.
Tag Description
User Guidance The medicinal product type shall be provided as applicable as a term ID.
• The applicable value(s) shall be selected from the term ID as listed in
the applicable Referentials Management Service (RMS) list.
• The value "Other" should be specified only if none of the other
available values is applicable.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000000324
Value(s)
The System and Code should be specified as listed in the XEVMPD
Medicinal Product Type RMS list
ISO Element Name
Product Classification
ISO Path
/MedicinalProduct/ProductClassification/
FHIR Element Name
productClassification
FHIR Path
MedicinalProductDefinition.productClassification
Example(s):
Medicinal product that is parallel distributed shall reference the value 'Parallel/Imported medicinal
product (Article 76(3) and (4) of Directive No 2001/83/EC)'.
1.13.2. Legal basis
The legal basis for the marketing authorisation shall be provided.
• For medicinal products where the legal basis of the marketing authorisation predates Directive
2001/83/EC, (taking into account that the pharmaceutical Acquis Communautaire has been
amended over time), the legal basis applicable under the current Union legal framework which
corresponds to the legal basis in the legislation that was applicable at time of submission of
your application shall be specified. This statement is in line with the xEVMPD requirement stated
in Note 5 of Chapter 3.II: XEVPRM User Guidance
.
• Where the authorisation procedure is specified as "EU Registration Procedures - Traditional use
registration for herbal medicinal product the legal basis should also be selected as "Traditional use
registration application for an herbal medicinal product (Article 16a of Directive No 2001/83/EC)".
• Where the authorisation procedure is specified as "EU Registration Procedures - Simplified
registration procedure for homeopathic medicinal products" the legal basis should also be selected
as "Simplified registration application for a homeopathic medicinal product (Article 14 of Directive
No 2001/83/EC)".

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 52/231
Tag Description
User Guidance The legal basis for the marketing authorisation shall be provided as
applicable as a term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
NOTE: The relevant RMS list contains terms which can be used for
medicinal products that can be submitted into PMS on a voluntary bases.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000116045
Value(s)
The System and Code should be specified as listed in the Marketing
Authorisation Application Legal Basis RMS list.
ISO Element Name
Product Classification
ISO Path
/MedicinalProduct/ProductClassification/
FHIR Element Name
productClassification
FHIR Path
MedicinalProductDefinition.productClassification
1.13.3. ATC code(s)
Tag Description
User Guidance • The ATC code as indicated in section 5.1 Pharmacodynamic properties
of the corresponding SmPC or other regulatory document shall be
provided (if available) as an RMS term ID. If multiple values apply to
the same medicinal product then multiple values shall be selected by
repeating the field.
• Deprecated (i.e., non-current) ATC Codes may be referenced.
• All five levels of an ATC Code can technically be used; however, the
most granular level of information is expected wherever available.
• The applicable value(s) shall be selected from the term ID as listed in
the applicable Referentials Management Service (RMS) list.
• If ATC code is not available or is not yet defined this field shall be
populated with the RMS ID referring to the substance group of the last
available level of the ATC Codes and information in 1.13.3.1 - ATC
Code flag completed.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000093533
Value(s)
Listed in the Anatomical Therapeutic Chemical classification system –
Human RMS list
ISO Element Name
Product Classification
ISO Path
/MedicinalProduct/ProductClassification
FHIR Element Name
productClassification
FHIR Path
MedicinalProductDefinition.productClassification

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 53/231
Example(s):
Section 5.1 of the SmPC states:
ATC code N03AX16 should therefore be selected from the RMS list; in RMS this will be uniquely
identified by a specific RMS ID (e.g., 100000093537).

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 54/231
1.13.3.1. ATC code(s) – Flag
Tag Description
User Guidance ATC Code - Flag shall be completed whenever ATC code is not available or
is not yet defined with the following values:
• True: Applicant or Marketing Authorisation holder applied for an ATC
code, but it has not yet been assigned.
• False: Applicant or Marketing Authorisation holder has not applied for
ATC code or ATC code is not applicable.
Repeatable
No
Conformance
Conditional
Data Type
Boolean
RMS URI/URL
Not applicable
Value(s)
True / False
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
atcPending
FHIR Path
MedicinalProductDefinition.productClassification.extension.atcPending
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
1.13.4. Medicinal product category
The medicinal product category based on the nature of the active substance or combination of
substances and based on how it exerts a pharmacological, immunological or metabolic action or
whether it has a special classification (e.g., advanced therapy medicinal product, vaccine, etc.) shall be
provided.
If multiple values apply to the same medicinal product, then multiple values shall be selected by
repeating the field (e.g., A vaccine consisting of a recombinant protein will be classified as
immunological and biological medicinal product).
Tag Description
User Guidance Indication on the type of medicinal product should be included to indicate
on whether the medicinal product is biological, vaccine, ATMP, chemical or
other product types based on the nature of active substance or
combination of substances.
The applicable value(s) shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
Yes
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000155526
Value(s)
Listed in the Product Category RMS list
ISO Element Name
Product Classification
ISO Path
/MedicinalProduct/ProductClassification
FHIR Element Name
productClassification
Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 55/231
Tag Description
FHIR Path
MedicinalProductDefinition.productClassification
Example(s):
Biological Medicinal Product, Immunological Medicinal Product, Advanced Therapy Medicinal Product,
Chemical Medicinal Product
1.13.5. Genetically Modified Organisms (GMOs)
In accordance with the definition provided in Article 2(2) of Directive 2001/18/EC:
“‘genetically modified organism (GMO)’ means an organism, with the exception of human beings, in
which the genetic material has been altered in a way that does not occur naturally by mating and/or
natural recombination.”
Therefore, the Genetically Modified Organisms (GMOs) data element is required to be specified with the
applicable value indicated in the below table.
Tag Description
User Guidance Genetically Modified Organisms attribute shall be completed to indicate
whether a medicinal product contain or consists of Genetically Modified
Organisms (GMOs) with the following values, based on the Directive
2001/18/EC:
• Yes: shall be selected if the medicinal product does contain or consist
of Genetically Modified Organisms (GMOs) within the meaning of
Directive 2001/18/EC
• No: shall be selected if the medicinal product does not contain or
consist of Genetically Modified Organisms (GMOs) within the meaning
of Directive 2001/18/EC
Repeatable
No
Conformance
Mandatory
Data Type
Boolean
RMS URI/URL
Not applicable
Value(s)
True / False
ISO Element Name
Product Classification
ISO Path
/MedicinalProduct/ProductClassification
FHIR Element Name
characteristic
FHIR Path
MedicinalProductDefinition.characteristic
1.14. Medicinal product name
The Medicinal product name is defined based on the following elements and structure:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 56/231
Figure 5: Extract of the Resource MedicinalProductDefinition (source: http://www.hl7.org/fhir)
The class is capturing the full medicinal product name in line with the country/language where the
name applies. The class NamePart is capturing fragments (coding words or phrases) of the medicinal
product name and is mandatory.
Note that the structure described above is implemented in a manner that does not allow the
submission of various name parts independently in various languages; once it is deemed necessary to
provide the name parts in a particular language, a minimum of mandatory fields (described below)
shall be provided. For example, it is not possible to enter only the ‘Intended use name part’ in German
and only the ‘Invented name part’ in French; the system will mandate for each language chosen a
minimum set of fields.
Product name and name parts should be provided using the capitalisation as stated in the SmPC.
The Medicinal product name class is mandatory and repeatable while the individual attributes of this
class are mandatory (with the exception of some name part data elements) and not repeatable (with
the exception of the delimiter part name data element).
Medicinal product name Class Description
Repeatable
Yes
Conformance
Mandatory
1.14.1. Full name
Tag Description
User Guidance The full medicinal product name as indicated in Section 1: Name of the
Medicinal Product of the corresponding SmPC or other regulatory
document shall be specified, in line with the local language of the country
where the product is authorised.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 57/231
Tag Description
• Full name, country and splitting of the name in parts shall be repeated
as per applicable languages in multi-language countries (e.g., Belgium)
• Based on the authorisation procedure, marketing authorisation holder
may submit translations of the name in English on an optional basis
(Refer to table 1a).
Repeatable
Yes
Conformance
Mandatory
Data Type
String
RMS URI/URL
Not applicable
Value(s)
The full medicinal product name as free text.
ISO Element Name
FullName
ISO Path
/MedicinalProduct/MedicinalProductName/FullName
FHIR Element Name
productName
FHIR Path
MedicinalProductDefinition.name.productName
Example(s):
Helia 200 mg compresse rivestite
BeatCold® Orange paracetamol easy-to-swallow tablets
Ibuprofen capsules
Diclofenac PharmaABC 32 Filmtabletten
DrugABC 2013/2014 suspension for injection (influenza vaccine, surface antigen, inactivated)
1.14.2. Country/Language
This section describes how to populate information on the language used for the product name, where
the medicinal product name shall be provided in additional official languages used in a country. The
class Country/Language is mandatory in the PMS implementation.
As a rule, it is mandatory when an SmPC/PL is available in a given language, the name and name parts
in that language shall be provided; this is captured in the table below under the phrasing ‘when
applicable’.
For the centrally authorised products, conventionally, the authorisation country should be filled in as
‘EU’.
The following table summarises the requirement:
Table 1a – Medicinal product – language requirements
Authorisation
Procedure
Country of
authorisation
Translations of medicinal product name and
source documentation: Summary of Product
Characteristics (SmPC)
Centralised
Procedure
(CAP)
EU Member states
English + All other languages as applicable in EU
Iceland, Liechtenstein
and Norway (IS/LI/NO)
English + National language(s) of the country of
authorisation when applicable

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 58/231
Authorisation
Procedure
Country of
authorisation
Translations of medicinal product name and
source documentation: Summary of Product
Characteristics (SmPC)
Mutual
Recognition
Procedure
(MRP)
EU Member state,
(IS/LI/NO)
National language(s) of the country of authorisation
Any other language when applicable
English translation – optional submission
Decentralised
Procedure
(DCP)
EU Member state,
(IS/LI/NO)
National language(s) of the country of authorisation
Any other language when applicable
English translation – optional submission
National
Procedure
(NAP)
EU Member state,
(IS/LI/NO)
National language(s) of the country of authorisation
Any other language when applicable
English translation – optional submission
English SmPC is acceptable where the local SmPC has not yet been issued; once the SmPC in the
national language becomes available it shall be provided in the context of the data maintenance; i.e.,
when the variation leads to changes.
List of official languages per country can be found on the EMA corporate website.
The Country/Language class and its attributes are mandatory and not repeatable.
Medicinal product name Class Description
Repeatable
No
Conformance
Mandatory
1.14.2.1. Country
Tag Description
User Guidance The country where the medicinal product name has been authorised, as
approved by the regulatory authority and indicated in the corresponding
regulatory document(s), shall be specified as a term ID.
• For products authorised through the MRP/DCP routes, the country of
the marketing authorisation shall be selected (products introduced
through this route are entered as individual product entities for each
national authorisation).
• For products authorised through purely national procedure, the
country where the marketing authorisation was granted shall be
selected)
• For products authorised through Centralised Procedure the value EU
shall be selected. Alternatively, Norway/Iceland/Liechtenstein shall be
selected as Norway/Iceland/ Liechtenstein are entered as individual
product entities.
At least one country shall be specified per Medicinal Product name.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 59/231
Tag Description
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000002
Value(s)
As listed in the Country RMS list.
ISO Element Name Country
ISO Path
/MedicinalProduct/MedicinalProductName/Country-Language/Country
FHIR Element Name
Country
FHIR Path
MedicinalProductDefinition.name.countryLanguage.country
Example(s):
France, Czech Republic, Ireland
1.14.2.2. Language
Tag Description
User Guidance The language of the medicinal product name for the specified country, as
approved by the regulatory authority and indicated in the corresponding
regulatory document(s) shall be specified as a term ID.
• The applicable value shall be selected from the term and term ID as
listed in the Referentials Management Service (RMS) list.
• For products authorised through MRP/DCP/National Procedure the
English translation of the name may be selected on a voluntary basis
(Refer to table 1a).
• For products authorised through MRP/DCP/National Procedure, in case
of countries with multiple languages (e.g., Belgium) the medicinal
product name in all official languages shall be specified (Refer to table
1b).
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072057
Value(s)
Listed in the Language RMS list
ISO Element Name
Language
ISO Path
/MedicinalProduct/MedicinalProductName/Country-Language/Language
FHIR Element Name
Language
FHIR Path
MedicinalProductDefinition.name.countryLanguage.language
Example(s):
French, English, Dutch

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 60/231
1.14.3. (Medicinal product name) name part(s)
The medicinal product name parts (MedicinalProductDefinition.name.namePart) shall be specified along
with the language of the country as shown in section 1.14.2. Country/Language and as the marketing
authorisation applies in accordance with the referenced SmPC.
List of official languages per country can be found on the EMA corporate website.
For additional related information please refer also to:
• Table 1a - Medicinal Product – language requirements (refer to section 1.14.2. Country/Language);
• Table 1b - Requirements for Medicinal Product records and attachments for countries with more
than one national language (refer to section 1.18. Attached document)
(Medicinal product name) name part(s) Class Description
Repeatable Yes – Delimiter part type
No – All other name part types
Conformance
Mandatory
The parts of the medicinal product name should be specified as follows:
1.14.3.1. Name part type
Tag Description
User Guidance The applicable value(s) shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list Medicinal Product
Name Part Type.
In accordance with the definition provided in Article 1(20) of Directive
2001/83/EC, the name of the medicinal product may be an invented name
or may be either the INN/common name, or a common or scientific name
accompanied by a trade mark or the name of the marketing authorisation
holder. Therefore, the following is required as minimum medicinal product
name:
• Either Type = Invented name part or/and Type = Scientific name part
shall be specified as applicable in the product name part;
• If Type = Scientific name part has been specified and the Type =
Invented name part has NOT been specified, the Type = Trademark or
company name part shall be specified as applicable
When the full presentation name along with its corresponding information
on ‘Country’ and ‘Language’ has been provided, it is expected that the
‘Invented Name Part’ is populated as minimum information. Where the
‘Invented name part’ is not applicable, both ‘Scientific Name part’ and
‘Trademark or company name part’ shall be populated as a minimum
requirement.
The system will reject the provision of name parts that do not comply with
these minimal requirements in order to ensure that the information

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 61/231
Tag Description
provided as regard to the medicinal product name is meaningful (e.g.,: it
is not accepted to provide only ‘Name’ and ‘Intended use part’ or only
‘Device part’ and ‘Flavour part’).
Repeatable
No
Conformance Mandatory
Data Type
Coding
RMS URI/URL https://spor.ema.europa.eu/v1/lists/220000000000
Value(s) The applicable name part type of the Full name as term listed in the
Referentials Management Service (RMS) list Medicinal Product Name Part
Type.
A dedicated RMS list is available with the following values:
• Invented name part
• Scientific name part
• Strength part
• Pharmaceutical dose form part
• Formulation part
• Intended use part
• Target population part
• Container or pack part
• Device part
• Trademark or company name part
• Time/period part
• Flavour part
•
Delimiter part
ISO Element Name (The types of the name parts from Medicinal Product Name)
ISO Path
/MedicinalProduct/Medicinal Product Name/{various}
FHIR Element Name
Type
FHIR Path
MedicinalProductDefinition.name.namePart.type
1.14.3.2. Name part text
Tag Description
User Guidance The fragment of a product name (as selected in 1.14.3.1) shall be
specified as applicable.
Repeatable
No
Conformance
Mandatory
Data Type
String
RMS URI/URL
Not applicable
Value(s)
The applicable name fragment of the Full name as free text
ISO Element Name
(The name parts from Medicinal Product Name)
ISO Path
/MedicinalProduct/MedicinalProductName/{various}
FHIR Element Name
Part
FHIR Path
MedicinalProductDefinition.name.namePart.part

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 62/231
1.14.3.3. Name part convention and guidance
1.14.3.3.1. Invented name part
Invented name part Description
Repeatable
No
Conformance
Conditional
The invented (i.e., trade) name part text, if contained within the Full name, shall be specified.
• If no invented name is included in the Full name, then no information needs to be provided in this
data element.
• If a trademark symbol (e.g., ®, ™) is included in the full name, it shall not be provided in this data
element. This information should be reported as trademark or company name part as indicated in
section.1.14.3.3.10. Trademark or company name part of this Chapter.
• With the scope to facilitate the precise identification of the product concerned for
pharmacovigilance purposes, if any qualifier [i.e., Plus, Zydis] is included in the full name, it shall
be provided in this data element.
This does not apply in case of any other characteristics included in the Full name (i.e., target
population, pharmaceutical dose form etc) to be reported in the designated name part(s) in the below
reported additional data elements.
Example(s):
Full name: Helia™ 200 mg compresse rivestite
Invented part: Helia
Full name: BeatCold® Orange paracetamol slow release tablets
Invented part: BeatCold
Full name: Ibuprofen capsules
Invented part: <this data element should not contain any information>
Full name: Diclofenac PharmaABC 32 Filmtabletten
Invented part: <this data element should not contain any information>
Full name: DrugABC 2013/2014 suspension for injection (influenza vaccine, surface antigen,
inactivated)
Invented part: DrugABC

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 63/231
Full name: XYZ Plus 20 Film-coated tablets
Invented part: XYZ Plus
Note: the examples above focus on the name part described and do not give a complete picture of all
the name parts applicable to each example
1.14.3.3.2. Scientific name part
Scientific name part Description
Repeatable
No
Conformance
Conditional
The scientific or common (i.e., generic) name part text, if contained within the Full name, shall be
specified. If no common (i.e., generic) name is included in the Full name, then no information shall be
specified in this data element.
With the scope to facilitate the precise identification of the product concerned for pharmacovigilance
purposes, if any qualifier [i.e., Plus, Zydis] is included in the full name, it shall be provided in this data
element. This does not apply in case of any other characteristics included in the Full name (i.e., target
population, pharmaceutical dose form etc) to be reported in the designated name part(s) in the below
reported additional data elements.
Example(s):
Full name: BeatCold® Orange paracetamol slow release tablets
Scientific part: paracetamol
Full name: Helia™ 200 mg compresse rivestite
Scientific part: <this data element should not contain any information>
Full name: Ibuprofen capsules
Scientific part: Ibuprofen
Full name: Diclofenac PharmaABC 32 Filmtabletten
Scientific part: Diclofenac
Full name: DrugABC 2013/2014 suspension for injection
Scientific part: <this data element should not contain any information>
Full name: Ibuprofen Zydis 40 Film-coated tablets
Scientific part: Ibuprofen Zydis

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 64/231
Note: the examples above focus on the name part described and do not give a complete picture of all
name parts applicable to each example.
1.14.3.3.3. Strength part
Strength part Description
Repeatable
No
Conformance
Conditional
The strength name part text, if contained within the Full name, shall be specified.
If no strength is included in the Full name, then no information shall be specified in this data element.
Note: The strength included in this field may be different from the strength of the active ingredient of
the pharmaceutical product.
Example(s):
Full name: Helia™ 200 mg compresse rivestite
Strength part: 200 mg
Full name: Ibuprofen 400 mg Liquid Capsules
Strength part: 400 mg
Full name: BeatCold® Orange paracetamol slow release tablets
Strength part: <this data element should not contain any information>
Full name: Diclofenac PharmaABC 32 Filmtabletten
Strength part: <this data element should not contain any information>
Full name: DrugABC 2013/2014 suspension for injection (influenza vaccine,
surface antigen, inactivated)
Strength part: <this data element should not contain any information>
Note: the examples above focus on the name part described and do not give a complete picture of all
name parts applicable to each example.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 65/231
1.14.3.3.4. Pharmaceutical dose form part
Pharmaceutical dose form part Description
Repeatable
No
Conformance
Conditional
The pharmaceutical dose form name part text, if contained within the Full name, shall be specified. If
no pharmaceutical dose form name is included in the Full name, then no information shall be specified
in this data element.
Note: The pharmaceutical dose form name included in this data element may be different from the
manufactured and/or administrable dose form.
Example(s):
Full name: Helia™ 200 mg compresse rivestite
Pharmaceutical dose form part: compresse rivestite
Full name: BeatCold® Orange paracetamol slow release tablets
Pharmaceutical dose form part: slow release tablets
Full name: Ibuprofen capsules
Pharmaceutical dose form part: capsules
Full name: Diclofenac PharmaABC 32 Filmtabletten
Pharmaceutical dose form part: Filmtabletten
Full name: DrugABC 2013/2014 (influenza vaccine, surface antigen,
inactivated)
Pharmaceutical dose form part: <this data element should not contain any information>
Full name: Superdrug Shower Gel
Pharmaceutical dose form part: Shower Gel
Note: the examples above focus on the name part described and do not give a complete picture of the
entire name parts applicable to each example.
1.14.3.3.5. Formulation part
Formulation part Description
Repeatable
No

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 66/231
Formulation part Description
Conformance
Conditional
The formulation name part text, if contained within the Full name, shall be specified. If no formulation
name is included in the Full Name, then no information shall be specified in this data element.
Example(s):
Full name: XYZ Strawberry 120 mg/5 ml sugar free oral suspension for children
Formulation part: sugar free
Full name: BeatCold® Orange paracetamol slow release tablets
Formulation part: <this data element should not contain any information>
Full name: DrugABC 2013/2014 suspension for injection (influenza vaccine,
surface antigen, inactivated)
Formulation part: <this data element should not contain any information>
Note: the examples above focus on the name part described and do not give a complete picture of all
the name parts applicable to each example.
1.14.3.3.6. Intended use part
Intended use part Description
Repeatable
No
Conformance
Conditional
The intended use name part text, if contained within the Full name, shall be specified.
If no intended use name is included in the Full name, then no information shall be specified in this data
element.
Example(s):
Full name: DrugABC 200mg migraine relief tablet
Intended use part: migraine relief
Full name: XYZ Strawberry 120 mg/5 ml oral suspension for children
Intended use part: <this data element should not contain any information>

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 67/231
Full name: ProductX Max Strength Heartburn Relief Oral Suspension
Intended use part: heartburn relief
Full name: Mivera contraceptive tablets
Intended use part: contraceptive
Note: the examples above focus on the name part described and do not give a complete picture of all
the name parts applicable to each example.
1.14.3.3.7. Target population part
Traget population part Description
Repeatable
No
Conformance
Conditional
The target population name part text, if contained within the Full name, shall be specified.
If no target population name is included in the Full name, then no information shall be specified in this
data element.
Example(s):
Full name: XYZ Strawberry 120 mg/5 ml oral suspension for children
Target population part: for children
Full name: ProductX Infant Suspension
Target population name: infant
Full name: ABC Adult Vaccine, suspension for injection
Target population part: adult
Full name: BeatCold® Orange paracetamol slow release tablets
Target population part: <this data element should not contain any information>
Note: the examples above focus on the name part described and do not give a complete picture of all
the name parts applicable to each example.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 68/231
1.14.3.3.8. Container or pack part
Container or pack part Description
Repeatable
No
Conformance
Conditional
The container or pack name part text, if contained within the Full name, shall be specified.
If no container or pack name is included in the Full name, then no information shall be specified in this
data element.
Example(s):
Full name: XYZ 50 mg/ml solution for injection in a vial
Container or pack part: in a vial
Full name: DrugABC 200mg migraine relief tablet
Container or pack part: <this data element should not contain any information>
Note: the examples above focus on the name part described and do not give a complete picture of all
the name parts applicable to each example.
1.14.3.3.9. Device part
Device part Description
Repeatable
No
Conformance
Conditional
The device name part text, if contained within the Full Name, shall be specified.
If no device name is included in the Full name, then no information shall be provided in this data
element.
Example(s):
Full name: ProductX 100 units/ml solution for injection in pre-filled pen
Device part: in pre-filled pen
Full name: DruxY 160 Inhaler
Device part: inhaler
Full name: XYZ Strawberry 120 mg/5 ml oral suspension for children

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 69/231
Device part: <this data element should not contain any information>
Note: the examples above focus on the name part described and do not give a complete picture of all
the name parts applicable to each example.
1.14.3.3.10. Trademark or company name part
Trademark or company name part Description
Repeatable
No
Conformance
Conditional
The trademark or company name part text, if contained within the Full name, shall be specified.
If no trademark or company name is included in the Full name, then no information shall be specified
in this data element except for cases where an invented name part is not mentioned in the full name.
In these exceptional cases the organisation name (MAH name) should be entered in order to give the
product name a minimum of identifiable product information.
Example(s):
Full name: Insulin PharmaX Comb 30/70 100 I.E.,/ml Zylinderampullen
mit Injektionssuspension
Trademark or company name part: PharmaX
Full name: XYZ® Express 200 mg Liquid Capsules
Trademark or company name part: ®
Full name: Ibuprofen liquid capsules
Trademark or company name part: PharmaABC
Note that Company name was not included in the name. In this exceptional case, the name of the
marketing authorisation holder shall be included (e.g., PharmaABC).
Full name: PharmaY Ibuprofen capsules
Trademark or company name part: PharmaY
Note: the examples above focus on the name part described and do not give a complete picture of all
the name parts applicable to each example.
1.14.3.3.11. Time/period part
Time/period part Description
Repeatable
No

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 70/231
Time/period part Description
Conformance
Conditional
The time/period name of the medicinal product part text, if contained within the Full name, shall be
specified.
If no time/period name is included in the Full name, then no information shall be specified in this data
element.
Example(s):
Full name: DrugABC 2013/2014 suspension for injection (influenza vaccine, surface
antigen, inactivated)
Time/period part: 2013/2014
Full name: Benny Allergy One A Day 20mg Tablets
Time/period part: One A Day
Note: the examples above focus on the name part described and do not give a complete picture of all
the name parts applicable to each example. Capitalisation/Format should be exactly as per the
attached regulated document (e.g., SmPC). Requirements for attached regulated documents can be
found in section 1.18. Attached document.
1.14.3.3.12. Flavour part
Flavour part Description
Repeatable
No
Conformance
Conditional
The flavour name part text, if contained within the Full name, shall be specified.
If no flavour name is included in the Full name, then no information shall be specified in this data
element.
Example(s):
Full name: BeatCold paracetamol Orange syrup
Flavour part: Orange
Full name: PharmaABC sore throat relief lozenges lemon flavour
Flavour part: lemon flavour

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 71/231
Note: the examples above focus on the name part described and do not give a complete picture of all
the name parts applicable to each example.
1.14.3.3.13. Delimiter part
Delimiter part Description
Repeatable
Yes
Conformance
Conditional
The delimiter name part text (including character such as /-(), and words including “and”), if contained
within the Full name, shall be specified. The delimiter is used to separate one composite in a segment
from another or separate one sub-composite from another.
Example(s):
Full name: Magraprime 5 mg / 15 mg tablets
Delimiter part: /
Full name: AmoAll (Amoxicillin 500 mg and Clavulanic acid 125 mg) tablets
Delimiter part: “And” ,“(” , “)”
1.15. (Pharmacovigilance) master file
The Pharmacovigilance master file location related to the authorised medicinal product shall be
specified. Other type of master files (active substance master file, vaccine master file, plasma master
file) are not currently in scope for this version of the EU IG.
The Pharmacovigilance system master file (PSMF) definition is provided in Article 1(28e) of Directive
2001/83/EC and the minimum requirements for its content and maintenance are set out in the
Commission Implementing Regulation (EU) No 520/2012 on the Performance of Pharmacovigilance
Activities Provided for in Regulation (EC) No 726/2004 and Directive 2001/83/EC (the Implementing
Regulation is referenced as IR).
The detailed requirements provided by the Commission Implementing Regulation are further supported
by the guidance in Module II – Pharmacovigilance system master file of the Good Vigilance Practice(s).
• The PSMF shall be located either:
− at the site in the EU where the main pharmacovigilance activities of the marketing
authorisation holder are performed; or
− at the site in the EU where the qualified person responsible for pharmacovigilance operates [IR
Art 7(1)].
As initial temporary process, registration of the PSMF location will need the use of two systems
XEVMPD and PMS and will be performed in two steps:
• (1) At the time of the marketing authorisation application, the applicant should submit
electronically the PSMF location information using the agreed format as referred to in chapter IV,
Article 26 of the Commission Implementing Regulation (EU) No 520/2012 using the XEVMPD

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 72/231
database and XEVPRM format. This will generate a unique PSMF location reference
number/identifier, which is the unique code assigned by the EMA to the master file.
• (2) Subsequently the PSMF location reference number/identifier generated in XEVMPD database
should be linked to the medicinal product during the submission of product data in PMS.
This process will be revised in future versions of the EU IG to make use of a central repository for
Master File information using SPOR capabilities.
The Pharmacovigilance system master file (PSMF) class and the individual attributes of this class are
mandatory and not repeatable.
Pharmacovigilance system master file
(PSMF) Class
Description
Repeatable
No
Conformance
Mandatory
1.15.1. File type
Tag Description
User Guidance The type of master file shall be specified.
• The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
• For iteration 1 implementation, the value “PharmacoVigilance System
Master File” shall always be specified.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/220000000070
Value(s) Pharmacovigilance Master File to be selected from
Master File Type RMS list.
ISO Element Name
File Type
ISO Path
/MedicinalProduct/MasterFile/FileType
FHIR Element Name
Type
FHIR Path DocumentReference.type (referenced from
MedicinalProductDefinition.masterFile)
Example(s):
Pharmacovigilance System Master File
1.15.2. File code
Tag Description
User Guidance
The Pharmacovigilance System Master File Location (PSMFL) reference
number/identifier generated by XEVMPD following successful registration in
XEVMPD database shall be specified in PMS

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 73/231
Tag Description
• The MFL EV Code is the unique code assigned by the XEVMPD to a
specific PSMF and to the location of the PSMF and it shall be specified
accordingly.
− If the Master File Location information was previously successfully
submitted in the XEVMPD and a PSMFL EV Code had been assigned,
the PSMFL shall be specified as available in the XEVMPD and as
received in the XEVPRM Acknowledgement.
− If the required Master File Location does not exist in the XEVMPD, the
Master File Location information can be added using the 'Master File
Location' section of the XEVPRM and submitted in the XEVMPD.
• Further information on what triggers the request of PSMFL EV Code and
how to submit MFL information in the XEVMPD are available in section
1.11. Initial submission of a Pharmacovigilance System Master File
(PSMF) information of the Detailed guidance on the electronic submission
of information on medicinal products for human use by marketing
authorisation holders to the European Medicines Agency in accordance
with Article 57(2) of Regulation (EC) No. 726/2004 - Chapter 3.II:
XEVPRM User Guidance.
• Information on how the PSMFL entity should be maintained is described
in section 2.3. Maintenance of a Pharmacovigilance System Master File
Location (PSMFL) entity of the Detailed guidance on the electronic
submission of information on medicinal products for human use by
marketing authorisation holders to the European Medicines Agency in
accordance with Article 57(2) of Regulation (EC) No. 726/2004 - Chapter
3.II: XEVPRM User Guidance.
This process will be revised in future versions of the EU IG to make use
of the Organisation Management System and PMS as repository for
Master File information.
Repeatable
No
Conformance
Mandatory
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
PSMFL as available in the XEVPMD/Article 57 database.
ISO Element Name
File Code
ISO Path
/MedicinalProduct/MasterFile/FileCode
FHIR Element Name
identifier
FHIR Path DocumentReference.identifier (referenced from
MedicinalProductDefinition.masterFile)
FHIR
Complementary
Information
DocumentReference.identifier.system value is
“https://spor.ema.europa.eu/v1/lists/100000000009/terms/100000075665”
Example(s):

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 74/231
EV Code assigned to the PSMFL entity in the Article 57 database: MFL1234
1.16. Contact (QPPV)
MAHs are legally required to have a qualified person for pharmacovigilance (QPPV) based in the
European Union (EU) in place at all times, in line with the Directive 2001/83/EC Article 104(3)(a).
Detailed information on how to register a QPPV can be obtained on the EudraVigilance registration
webpage or in the EMA EudraVigilance Registration Manual.
The current registration of the QPPV is performed in two steps:
(1) QPPVs are required to self-register in the EMA Account Management Platform. Following the self-
registration of the QPPV in the EMA Account Management Platform the QPPVs submit an EMA Service
Desk request requesting their role to be certified by the EMA. Once the role is approved by the EMA,
the QPPV retrieves the QPPV Code assigned.
(2) The QPPV is required to complete the registration information in the EudraVigilance restricted area.
Upon completion of the registration is, the QPPV code from EudraVigilance shall be referenced in
PMS.
The details of the QPPV are pre-registered externally to PMS, therefore it is not necessary to provide
full details of the QPPV again in the submission of the product. A reference to the identifier of the QPPV
code of the pre-registered QPPV shall be used instead.
Figure 6: Extract of the Contact (QPPV) resource (source: http://www.hl7.org/fhir
)
Contact (QPPV) class and its individual attributes are mandatory and not repeatable.
Contact (QPPV) Class Description
Repeatable No
Conformance Mandatory

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 75/231
1.16.1. Identifier
Figure 7: Extract of the Contact (Practitioner Role) resource (source: http://www.hl7.org/fhir)
Tag Description
User Guidance The identifier of the QPPV code as received after self-registration in the
EMA Account Management Platform and completing the registration in
EudraVigilance shall be specified. Please refer to the information available
on the EudraVigilance: how to register webpage, section 'Registering
individual users'.
Repeatable
No
Conformance
Mandatory
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
The unique identifier of the QPPV shall be provided along with the
reference to the system that issues it (EMA Account Management Platform)
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Identifier
FHIR Path
MedicinalProductDefinition.contact.contact(PractitionerRole).identifier
FHIR Complementary
Information
MedicinalProductDefinition.contact.contact(PractitionerRole).identifier.syste
m value to be defined based on RMS term entry for Source of Information
RMS list.
Example(s):
1234

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 76/231
1.16.2. Role
Figure 8: Extract of the Contact resource (source: http://www.hl7.org/fhir)
Tag Description
User Guidance The type of the contact in the context of the Medicinal Product shall be
specified.
For each medicinal product the type QPPV shall be specified. Only one
QPPV can be specified per each medicinal product.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000154441
Value(s) The value “Qualified Person in the EEA for Pharmacovigilance” shall be
selected from the Contact Party Role RMS list.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Type
FHIR Path
MedicinalProductDefinition.contact.type
Example(s):
Qualified Person in the EEA for Pharmacovigilance (100000155057)

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 77/231
1.17. Pharmacovigilance enquiry information
Figure 9: Extract of the Contact (Organization) resource (source: http://www.hl7.org/fhir
)
The Pharmacovigilance enquiry information and its individual attributes are mandatory and repeatable,
however one set of information is recommended to be specified.
Pharmacovigilance enquiry information Class Description
Repeatable
Yes
Conformance
Mandatory
1.17.1. Email address
The information on Pharmacovigilance enquiry contacts is mandatory and shall be provided by means
of the Contact Resource using the same representation described in section 1.16. Contact (QPPV).
Figure 10: Extract of the detailed Contact (Organization) resource (source: http://www.hl7.org/fhir)
Tag Description
User Guidance The contact information for Pharmacovigilance enquires (functional e-mail
and phone where enquiries related to Pharmacovigilance for each
medicinal product shall be specified) will be made public by the Agency.
This field carries the functional email address.
Repeatable
No
Conformance
Mandatory
Data Type
ContactPoint
RMS URI/URL
Not applicable
Value(s)
A valid email address.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Telecom

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 78/231
Tag Description
FHIR Path
MedicinalProductDefinition.contact.contact(Organization).contact.telecom
Example(s):
system=email, value=pharmacovigilance@acme.com
1.17.2. Phone number
Figure 11: Extract of the detailed Contact (Organization) resource (source: http://www.hl7.org/fhir)
Tag Description
User Guidance The contact information for Pharmacovigilance enquiry (functional e-mail
and phone where enquiries related to Pharmacovigilance for each
medicinal product shall be specified) will be made public by the Agency.
This field carries the phone number.
Repeatable
No
Conformance
Mandatory
Data Type
ContactPoint
RMS URI/URL
Not applicable
Value(s)
A valid phone number with international prefix.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Telecom
FHIR Path
MedicinalProductDefinition.contact.contact(Organization).contact.telecom
Example(s):
system=phone, value=+311234567890

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 79/231
1.17.3. Role
Figure 12: Extract of the Contact resource (source: http://www.hl7.org/fhir)
Tag Description
User Guidance The type of the contact in the context of the Medicinal Product shall be
specified, indicating that this contact is of type pharmacovigilance enquiry
information.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000154441
Value(s) The value “Pharmacovigilance Enquiry Information” shall be selected from
the Contact Party Role RMS list
ISO Element Name
Role
ISO Path /MedicinalProduct/MarketingAuthorisation/MarketingAuthorisationHolder/C
ontact/Role
FHIR Element Name
Type
FHIR Path
MedicinalProductDefinition.contact.type
1.18. Attached document
The medicinal product shall reference at least one document(s) supporting the regulatory process
(e.g., initial marketing authorisation, variation, renewal, transfer of marketing authorisation etc.).
A copy of the SmPC document as authorised by the authorising body shall be provided. If this is not
available, another equivalent document such as a package leaflet shall be provided.
i.e.,Note 1: The common contents of each document [i.e., Module 1.2 – Electronic Application form
(eAF), Relevant sections in Module 3 – Quality, Summary of Product Characteristics (SmPC)]
supporting the regulatory process shall be aligned where applicable to ensure the discrepancies across
the documents are minimized. The content should enhance the quality of the product data reported in
Product Management Service (PMS). This requirement applies to new medicinal products single entry
in PMS. Refer to Submission of medicinal product data using FHIR.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 80/231
Table 1b - Requirements for medicinal product records and attachments for countries with
more than one national language
Country National
language(s)
Different
MPID
assigned?
Attachme
nt to be
used for
reference
Comment Language to
be used when
specifying
medicinal
product name
and its
applicable
name part(s)
Belgium
Dutch
No
SmPC
Dutch
French
No
SmPC
French
German No PL Since there is no
SmPC in German, the
PL is to be used
The document granting
authorisation/renewal
should also be
provided if the
authorisation number
or equivalent identifier
is not stated in the
referenced PL
German
Finland
Finnish
No
SmPC
Finnish
Swedish No SmPC or
PL
In cases where there is
no SmPC in Swedish,
the PL is to be used
The document granting
authorisation/renewal
should also be
provided if the
authorisation number
or equivalent identifier
is not stated in the
referenced PL
Swedish
Ireland
English
No
SmPC
English
Irish No n/a Authorisations are not
issued in Irish and no
SmPC/PL exists in this
language
n/a
Luxembourg* French No SmPC or
an
equivalent
document
(e.g., PL or
similar text
as
authorised
French or Belgian
SmPC/PL in French can
be used
The document granting
authorisation/renewal
should also be
provided if the
authorisation number
French

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 81/231
Country
National
language(s)
Different
MPID
assigned?
Attachme
nt to be
used for
reference
Comment
Language to
be used when
specifying
medicinal
product name
and its
applicable
name part(s)
by the
Authorisin
g Body)
or equivalent identifier
is not stated in the
referenced SmPC/PL
German No SmPC or
an
equivalent
document
(e.g., PIL
or similar
text as
authorised
by the
Authorisin
g Body)
German, Austrian or
Belgian SmPC/PL in
German can be used
The document granting
authorisation/renewal
should also be
provided if the
authorisation number
or equivalent identifier
is not stated in the
referenced SmPC/PL
German
Luxembourgis
h
No n/a Authorisations are not
issued in
Luxembourgish and no
SmPC/PL exists in this
language
n/a
Malta English No SmPC The document granting
authorisation/renewal
should also be
provided if the
authorisation number
or equivalent identifier
is not stated in the
referenced SmPC
English
Maltese No n/a Authorisations are not
issued in Maltese and
no SmPC/PL exists in
this language
n/a
* Table 1b covers the scenario when the French as well as the German SmPC/PL are provided to the
Luxembourgish Authority. If the MAH decided to provide only the French SmPC/PL or only the German
SmPC/PL to the Luxembourgish Authority, then only one applicable document should be submitted in
PMS.
Where, in exceptional circumstances, the national SmPC for non-centrally authorised products
(MRPs/DCPs/NAPs) is not available, a similar text (i.e., the English common text, package leaflet or
other similar text as authorised by the Authorising Body) can be used as an attachment for the
submission in PMS. The medicinal product name and its applicable name part(s) shall however be
provided in the language of the country where the marketing authorisation applies.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 82/231
In addition, the following documents from module 1 and module 3 of the Common Technical Dossier
(CTD) dossier shall be included where available (e.g., first submissions, changes in the composition of
the medicinal product, changes/introductions of manufacturers of the finished product or active
substance):
• Module 1.2 – Electronic Application form (eAF) (The eAF is the only document in Module 1.2
required, to be provided where relevant);
• Module 3.2.S.2.1 Manufacturer(s) (name, dosage form);
• Module 3.2.P.1 Description and Composition of the Drug Product (name, dosage form);
• Module 3.2.P.3.1 Manufacturer(s) (name, dosage form);
For Centrally Authorised products only the English version is required.
Document File type and format
The allowed file types for printed product information (i.e., SmPC/PL/marketing authorisation decision)
and module 3 of the CTD dossier are: .PDF (1) [format: PDF v.1.4 and above, preferable PDF/A], .DOC
(2), .DOCX (3).
Note: Scanned documents uploaded in PDF format should be avoided.
The FHIR DocumentReference (Figure 6
) resource is used to describe documents. In the context of
Iteration 1 of the PMS implementation, the information in figure 6 highlighted in red is within the scope
and should be provided according to the rules and guidance described in this section:
Figure 13: Resource DocumentReference (source : http://www.hl7.org/fhir)
The Attachment document class is mandatory and repeatable while the individual attributes of this
class are not repeatable (except for the alternative (Attached document) Identifier data element) and
shall be populated as applicable.
Attachment document Class Description
Repeatable
Yes

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 83/231
Attachment document Class Description
Conformance
Mandatory
1.18.1. Master (Attached document) Identifier
This section allows the MAH to indicate the identifier generated by the PMS system once the applicable
attached document is uploaded into PMS.
The Attached document Identifier Section is completed in two steps:
(1) The intended document is directly uploaded into PMS. Once uploaded, PMS assigns a document
identifier automatically and this information is available for the user (Refer to API Specification
(Chapter VI) for further information).
(2) The user should record the PMS Attached Document Identifier during the submission on Medicinal
Product Data.
The use of this data elements is based on the established processes of document submission discussed
in EU IG Chapter 3 - Process for the electronic submission of medicinal product information (Stepwise
approach for PMS Implementation).
The Master (Attachment document) Identifier attributes are conditional and not repeatable.
Attachment document Class Description
Repeatable
No
Conformance
Conditional
1.18.1.1. Identifier value
Tag Description
User Guidance The ID assigned to the document once it is uploaded to the PMS system,
which refers to the medicinal product, shall be specified. This identifier is
specific to this version of the document. This unique identifier may be used
elsewhere to identify this version of the document.
Refer to Annex 9.1.1 of SPOR API technical specifications v2
Not applicable in the case of Nationally Authorised Products during Step 1
of implementation of the TOM, unless the medicinal product data is chosen
to be submitted via API.
Repeatable No
Conformance
Mandatory
Data Type
String
RMS URI/URL
Not applicable
Value(s)
Relevant identifier assigned by PMS once document is uploaded
ISO Element Name
Identifier
ISO Path
/MedicinalProduct/AttachedDocument/Identifier
FHIR Element Name
value
FHIR Path DocumentReference.identifier.value (referenced from
MedicinalProductDefinition.attachedDocument)

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 84/231
1.18.1.2. Identifier system
Tag Description
User Guidance The source system relevant to the attached documentation can be
specified.
The term “Product Management Service” shall be provided as a term ID
from the Source of Information RMS list.
Repeatable
No
Conformance
Mandatory
Data Type
URI
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000009
Value(s) URI referencing to the applicable RMS term from the Source of Information
RMS list (i.e., https://spor.ema.europa.eu/v1/lists/100000000009
/terms/100000075665).
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
System
FHIR Path DocumentReference.identifier.system (referenced from
MedicinalProductDefinition.attachedDocument)
Example(s):
- Product Management Service (PMS) [200000024893]
1.18.2. Alternative (Attached document) Identifier
This section allows the MAH to indicate whether the attached document has any other identifier in
XEVMPD or any other external identifier that is used to identify the attached document, in addition to
the referring source system.
This data elements can be used either to complete the information in addition to the 1.19.1. Master
(Attached document) identifier or alternatively when information on 1.19.1. is not available.
The use of this data elements is based on the established processes of document submission discussed
in EU IG Chapter 3 - Process for the electronic submission of medicinal product information (Stepwise
approach for PMS Implementation). The Alternative (Attachment document) Identifier attribute is
optional and repeatable.
Attachment document Class Description
Repeatable Yes
Conformance
Optional
1.18.2.1. Identifier value
Tag Description
User Guidance The ID assigned to the document by another source system.
The EV Code of the attachment referring to the authorised medicinal
product may be specified if applicable.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 85/231
Tag Description
• If the attachment to be referenced was already submitted in the
XEVMPD and an attachment EV Code has been assigned,
• If the corresponding attachment is not available in the XEVMPD, the
attachment identifier of another external system can be provided (i.e.,
applicant’s document identifier in case the attachment is available in
the industry’s database and an identifier is assigned to it).
• If the corresponding attachment is provided via the eCTD link, the link
path to the attachment can be provided in this data element
Repeatable
No
Conformance
Mandatory
Data Type
String
RMS URI/URL
Not applicable
Value(s) The pattern of the EV Code is 'ATT' followed by a number or alternatively
the eCTD path.
In the context of the migration of the Article 57 data from the XEVMPD
into PMS, this information will be automatically provided in PMS as
described in Chapter 7 of this Guidance.
ISO Element Name
Identifier
ISO Path
/MedicinalProduct/AttachedDocument/Identifier
FHIR Element Name
value
FHIR Path DocumentReference.identifier.value (referenced from
MedicinalProductDefinition.attachedDocument)
Example(s):
ATT12345
1.18.2.2. Identifier system
Tag Description
User Guidance
The source system relevant to the attached documentation identifier can
be specified. For each specified value can correspond only one source
system.
• If the attachment identifier is the EV Code assigned in xEVMPD, the
“Extended EudraVigilance Medicinal Product Dictionary” is to be
selected,
• If the attachment identifier is referring to another external system
such as the internal industry database, the term “Applicant’s
document” is to be selected
• If the attachment is provided via the eCTD link and the link path to the
attachment can be provided in this data element, the term “electronic
Common Technical Document” is to be selected
Repeatable
No
Conformance
Mandatory
Data Type
URI

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 86/231
Tag Description
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000009
Value(s) URI referencing to the applicable RMS term from the Source of Information
RMS list (i.e., https://spor.ema.europa.eu/v1/lists/100000000009
/terms/100000075665)
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
System
FHIR Path DocumentReference.identifier.system (referenced from
MedicinalProductDefinition.attachedDocument)
Example(s):
- Extended EudraVigilance Medicinal Product Dictionary (100000075665);
- electronic Common Technical Document (100000153730)
- Applicant’s document (200000025171)
1.18.3. (Attached document) Type
Tag Description
User Guidance The value indicating the type of document shall be specified as a term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
In case where the approved SmPC does not state an authorisation
number, a date of authorisation/renewal or the MAH, a copy of the
document granting or renewing marketing authorisation (i.e., Letter of
authorisation) should also be provided as an additional attachment.
This list is to be updated with additional required values.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL • https://spor.ema.europa.eu/v1/lists/100000155531
• https://spor.ema.europa.eu/v1/lists/100000155719
•
https://spor.ema.europa.eu/v1/lists/100000155552
Value(s) As applicable in one the following RMS lists:
• Product Information Document Type: Summary of Product
Characteristics; Package leaflet eCTD EU Context of Use: M1.2
Application Form; M3.2.P.1 Description and Composition of the Drug
Product (name, dosage form); M3.2.P.3.1 Manufacturer(s) (name,
dosage form); M3.2.S.2.1 Manufacturer(s) (name, manufacturer)
• Regulating Authority Submission Unit Type: Authorisation letter
• Notification from a Competent Authority of the end/approval of
regulatory procedure
ISO Element Name
Type
ISO Path
/MedicinalProduct/AttachedDocument/Type
FHIR Element Name
Type

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 87/231
Tag Description
FHIR Path DocumentReference.type (referenced from
MedicinalProductDefinition.attachedDocument)
1.18.4. (Attached document) Effective Date
Tag Description
User Guidance The date, by which the corresponding updates become effective, as
specified in the attached reference document shall be specified once
available.
• Where the version date is reflected in the SmPC (i.e., the physical
document), it should be reflected as presented in section 10. Date of
revision of text of the SmPC.
• When the date is not stated in the physical document, the date when
the SmPC/module 1/module 3 documents have been approved by the
NCA can be provided.
• For quality changes not requiring regulatory approval to be included in
the terms of the marketing authorisation (e.g., Type IA variations), the
date of the implementation of this change should be provided.
Repeatable
No
Conformance
Conditional
Data Type
Period
RMS URI/URL
Not applicable
Value(s)
A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month, should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
Effective Date
ISO Path
/MedicinalProduct/AttachedDocument/EffectiveDate
FHIR Element Name
Date
FHIR Path DocumentReference.context.period.start (referenced from
MedicinalProductDefinition.attachedDocument)
1.18.5. (Attached document) Language
Tag Description
User Guidance The language used in the attached document(s) of the specified country
shall be specified as term ID.
• The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
Yes
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL https://spor.ema.europa.eu/v1/lists/100000072057

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 88/231
Tag Description
Value(s)
Listed in the Language RMS list
ISO Element Name
Language
ISO Path
/MedicinalProduct/AttachedDocument/Language
FHIR Element Name
valueCodeableConcept
FHIR Path DocumentReference.content.extension.language(referenced from
MedicinalProductDefinition.attachedDocument)
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
Example(s):
French (100000072175), English (100000072147)
1.18.6. URL value (New)
Tag Description
User Guidance
The URL of the document as identifiable in the Document API of PMS
Repeatable
No
Conformance
Mandatory
Data Type
url
RMS URI/URL
Not applicable
Value(s)
REST endpoint URL based on https://spor.ema.europa.eu/v*/documents
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
url
FHIR Path DocumentReference.content.attachment.url(referenced from
MedicinalProductDefinition.attachedDocument)
1.18.7. (Attached document) Status (New)
Tag Description
User Guidance This field is mandatory in the FHIR specification. The value ‘current’ shall
be used always.
Repeatable
No
Conformance
Mandatory
Data Type
code
RMS URI/URL
Not applicable
Value(s)
Hardcoded to ‘current’
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
status

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 89/231
Tag Description
FHIR Path DocumentReference.status (referenced from
MedicinalProductDefinition.attachedDocument)
1.19. Product cross-reference
A cross-reference to one or more medicinal products is to be made, if applicable, using this section.
The section applies to the following scenarios:
1. If a medicinal product has been authorised under the following legal basis (or equivalent previous
legislation):
• Generic application (Article 10(1) of Directive No 2001/83/EC);
• Hybrid application (Article 10(3) of Directive No 2001/83/EC)
• Similar biological application (Article 10(4) of Directive No 2001/83/EC);
• Informed consent application (Article 10(c) of Directive No 2001/83/EC);
the PMS ID of the innovator medicinal product should be provided.
In addition, duplicate applications of any legal basis submitted under Article 82(1) of Regulation
(EC) No 726/2004 should also be cross-referenced.
2. If a medicinal product is a Parallel Imported medicinal product (based on Article 76(3)), the PMS
ID of the following related products may be provided as follows:
• Source medicinal product of Parallel Import: medicinal product authorised in a different country
acting as source of the imported medicinal product in the destination country.
• Reference medicinal product of Parallel Import: medicinal product already authorised in the
destination country and which serves as a reference for the parallel imported product intended
to be imported into the destination country.
If the above scenarios apply for medicinal products, product cross-reference should be made even if
the originator product has been withdrawn of the market.
In the context of the iteration 1 implementation, only PMS ID can be referenced in this data element
and Investigational Medicinal Product Identifiers (including EV Codes as assigned by the XEVMPD
system for development products) are out of scope.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 90/231
Figure 14: Extract of the Product cross-reference resource (source: http://www.hl7.org/fhir)
The Product cross-reference class including the individual attributes are conditional and not repeatable.
Product cross-reference Class Description
Repeatable
Yes
Conformance
Conditional
1.19.1. Product cross-reference type
Tag Description
User Guidance If applicable, the type of medicinal product that is referenced shall be
specified as a term ID.
The applicable value shall be selected from the term ID as listed in the
Referentials Management Service (RMS) list.
This list is to be updated with additional required values.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/220000000017
Value(s)
As listed in the Product Cross Reference Type RMS list.
ISO Element Name
Referenced Product Type
ISO Path
/MedicinalProduct/ProductCross-Reference/ReferencedProductType
FHIR Element Name
Type
FHIR Path
MedicinalProductDefinition.crossReference.type
Example(s):
Generic of (220000000020), Biosimilar to (220000000018), Reference medicinal product of parallel
import (200000026060), Source medicinal product of parallel import (200000026059)

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 91/231
1.19.2. Product cross-reference resource identifier
The Product Cross-Reference class is conditional. Should the Product Cross-Reference class be
applicable, the following guidance applies:
Tag Description
User Guidance The PMS ID of the medicinal product that is referenced shall be provided.
This applies to products under specific legal basis (e.g., Generic, Hybrid,
Biosimilar) as reflected in introduction to section 1.19. Product cross-
reference.
Any reference in the resource identifier cross-reference section shall not be
a nullified version of the medicinal product.
This section is mandatory in case that product reference type is selected.
Repeatable
No
Conformance
Mandatory
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
PMS ID
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
productReference
FHIR Path
MedicinalProductDefinition.crossReference.productReference
Example(s):
PMS ID: 00005005
PMS ID: 00009105
1.20. Manufacturing business operation
This section describes how to populate information related to manufacturing sites and their operations.
This includes all activities performed by the manufacturing site involved in the processing of a
medicinal product or an active substance e.g., manufacturing, quality control, packaging and batch
certification.
This section shall be completed for each individual manufacturing site that performs any operation with
regards to the manufacturing of the finished product as reflected in module 3.2.P.3.1, and of the active
substance as reflected in 3.2.S.2.1. The ‘Manufacturing Business Operation’ class is mandatory in the
context of PMS implementation. This section is repeatable depending on the number of operation types
to include. If a manufacturing site performs more than one operation type with regards to the
manufacturing of the finished product and of the active substance, this section shall be repeated to
report the relevant information.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 92/231
Figure 15: Extract of Manufacturing Business Operation resource (source: http://www.hl7.org/fhir)
The Manufacturing business operation class is mandatory and repeatable while the individual attributes
of this class are not repeatable and shall be populated as applicable.
Manufacturing business operation Class Description
Repeatable
Yes
Conformance
Mandatory
1.20.1. Manufacturer
Tag Description
User Guidance The Manufacturer shall be specified using the location identifier (LOC ID)
linked to the organization as listed in the Organisation Management
System (OMS) following a successful registration of the organisation’s
details.
If the required organisation and/or related location are not available, the
addition of the unlisted organisation and/or related location should be
requested from OMS using the process described in the OMS Web User
Manual in the 'Documents' section of the Organisation Management
System (OMS).
When the LOC ID is specified, the system would give the information to
which ORG ID the selected location is linked in the back end. This
information will be shown through the PMS User Interface.
Repeatable
No
Conformance
Mandatory
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
As listed in SPOR OMS service (LOC ID)
ISO Element Name
Manufacturer(Organisation)
ISO Path MedicinalProduct/ManufacturerEstablishmentOrganisation/Manufacturer(Or
ganisation)

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 93/231
Tag Description
FHIR Element Name
manufacturingBusinessOperation
FHIR Path ActivityDefinition.participant.extension.manufacturingBusinessOperation
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
1.20.2. Operation type
This section describes the operation(s) being performed by the manufacturing site for a medicinal
product (including activities related to the manufacture of the active substance as applicable).
Operations to be selected should be in line with the information included in section 2.5 - Manufacturers
of the initial Marketing Authorisation Application electronic application form (in case of initial
submission) and module 3.2.P.3.1 / 3.2.S.2.1 – Manufacturers (in case of changes to the terms of the
marketing authorisation).
This should include manufacturing sites of any diluent/solvent presented in a separate container, but
forming part of the medicinal product, quality control/in-process testing sites, immediate (primary) and
outer (secondary) packaging as well as importer(s).
In addition, all manufacturing sites involved in the manufacturing process of the active substance,
including quality control/ in-process testing sites should be listed. For biotech products include all sites
of storage of master and working cell bank and preparation of working cell banks should also be
included.
For details on the applicable manufacturing operations See Compilation of Union Procedures on
Inspections and Exchange of Information document, (see sections Interpretation of the Union Format
for Manufacturer/Importer Authorisation and of the Union Format for GMP certificate).
Tag Description
User Guidance The type of manufacturing operation shall be specified as a term ID.
The applicable value shall be selected from the term ID as listed in the
Manufacturing Activity Referentials Management Service (RMS) list.
The applicable manufacturing operation(s) to be completed as per section
2.5 of the initial Marketing Authorisation eAF.
Manufacturing operations of finished product and/or active substance
(include manufacturers of intermediates of the active substance) to be
included as applicable.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000160406
Value(s)
Listed in the Manufacturing Activity RMS list
ISO Element Name
Operation Type
ISO Path /MedicinalProduct/ManufacturerEstablishmentOrganisation/ManufacturingB
usinessOperation/OperationType
FHIR Element Name
code
FHIR Path
ActivityDefinition.code

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 94/231
Example(s):
Primary packaging, Processing of sterile medicinal product – terminally sterilised,
Quality control testing – Biological,
Manufacture of active substance intermediate by chemical synthesis
1.20.3. Manufacturing operation start date
Tag Description
User Guidance The date when the manufacturing operation is approved/included in the
terms of the marketing authorisation of the medicinal product, shall be
provided:
• For manufacturing operations requiring regulatory approval to be
included in the terms of the marketing authorisation, the date of
finalisation of the applicable regulatory procedure should be provided.
In this case, this data field can only be populated with the necessary
information upon end of the regulatory procedure as this information
will not be available at the time of the submission of the FHIR message
(initial sequence).
• For manufacturing operations not requiring regulatory approval to be
included in the terms of the marketing authorisation (e.g., Type IA
variations), the date of the implementation of this change in the
Quality System should be provided.
Repeatable
No
Conformance
Conditional
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s)
A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
Not Applicable
ISO Path
Not Applicable
FHIR Element Name
start
FHIR Path ActivityDefinition.effectivePeriod.start
1.20.4. Manufacturing operation end date
Tag Description
User Guidance The date when the manufacturing operation is discontinued, and
consequently removed from the terms of the marketing authorisation of
the medicinal product shall be provided:
• For manufacturing operations not requiring regulatory approval to be
included in the terms of the marketing authorisation (e.g., Type IA

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 95/231
Tag Description
variations), the date of the implementation of this change in the
Quality System should be provided.
• For manufacturing operations requiring regulatory approval to be
included in the terms of the marketing authorisation, the date of
finalisation of the applicable regulatory procedure should be provided.
Repeatable
No
Conformance
Conditional
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s)
A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e.,, YYYY-MM).
ISO Element Name
Not Applicable
ISO Path
Not Applicable
FHIR Element Name
end
FHIR Path ActivityDefinition.effectivePeriod.end
1.20.5. Confidentiality indicator
Confidentiality indicator shows whether the manufacturing operation performed by a manufacturing
site is classified as confidential or public information. This information shall be associated with a
manufacturing operation.
Manufacturer information and its link to specific medicinal products that are included in the public
domain (e.g., batch release site, manufacturer of active substance for biological products) as
mandated by the current regulatory framework shall be classified as public/non-restricted information.
Remaining manufacturing operations are classified as confidential, therefore are not accessible to the
public.
Confidentiality is completed at the level of the manufacturing operations being performed by a given
manufacturer. E.g., Manufacturer ABCD may perform primary packaging and batch release operations
for a given product. In this case, the operation/activity batch release is specified as public information
whereas primary packaging is specified as confidential information.
Tag Description
User Guidance The type of level of confidentiality of the manufacturing operation should
be specified as Confidential/Public information
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list Data Classification.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 96/231
Tag Description
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000004983
Value(s)
The value(s) “Confidential” or “Public” shall be selected from the term ID
as listed in the Data Classification RMS list.
ISO Element Name
Confidentiality Indicator
ISO Path /MedicinalProduct/ManufacturerEstablishmentOrganisation/ManufacturingB
usinessOperation/ConfidentialityIndicator
FHIR Element Name
confidentialityIndicator
FHIR Path MedicinalProductDefinition.manufacturingBusinessOperation.confidentiality
Indicator
Example(s):
Confidential (200000004984), Public (200000004985)
1.20.6. Manufacturing authorisation reference number
The reference number of the authorisation issued by the relevant Medicines Regulatory Agency
(national competent authority) for the manufacturing of medicinal products shall be selected.
• If the manufacturing site is in the EEA: Reference Number of the Manufacturing and Importation
authorisation (MIA).
• If manufacturing site is located outside the EEA: Reference number of the equivalent of the
manufacturing authorisation.
• If manufacturing site is located outside the EEA and the site has been inspected by an EEA
authority: reference number of the relevant GMP certificate.
Tag Description
User Guidance The reference number of the authorisation for manufacturing or its
equivalent as provided by the medicines regulatory agency (national
competent authority), shall be specified.
• If manufacturing site is located in the EEA: Authorisation number to
be extracted from section 1 of the Union format for Manufacturer’s
Authorisation or from “MIA number” column in EudraGMDP.
• If manufacturing site is located outside the EEA: Reference number
of the equivalent of the manufacturing authorisation.
• If manufacturing site is located outside the EEA and the site has
been inspected by an EEA authority: Authorisation number is to be
extracted from section “Certificate number” within the certificate or
from “Certificate number” column in EudraGMDP.
Repeatable
No
Conformance
Conditional
Data Type
Identifier
RMS URI/URL
Not applicable

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 97/231
Tag Description
Value(s)
Reference Number
ISO Element Name
Manufacturing Authorisation Reference Number
ISO Path /MedicinalProduct/Manufacturer-
Establishment_Organisation/Manufacturing-
BusinessOperation/ManufacturingAuthorisationReferenceNumber
FHIR Element Name
identifier
FHIR Path RegulatedAuthorization.identifier (subject reference to relevant
ActivityDefinition)
FHIR Complementary
Information
RegulatedAuthorization.identifier.system value is
“http://ema.europa.eu/fhir/manufacturingAuthorizationNumber”
Example(s):
Manufacturing Authorisation certificate number
482927, 0000000069/19/1
GMP certificate reference numbers
1.20.7. Effective date
Tag Description
User Guidance The effective date of the Manufacturing Authorisation as stated in the
authorisation should be indicated if known.
If manufacturing site is located in the EEA: Effective date to be extracted
from section 9 of the Union format for Manufacturer’s Authorisation or
from “Authorisation Date” column in EudraGMDP.
If manufacturing site is located outside the EEA:
• Effective date extracted from the equivalent applicable authorisation
issued by the local authority.
• Effective date extracted from the GMP certificate issue by an EEA
authority or from column “Inspection End Date” in EudraGMDP.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 98/231
Tag Description
• This value is an attribute within Manufacturing Authorisation Reference
Number.
Repeatable
No
Conformance Conditional (if Manufacturing Authorisation Reference Number is
introduced and effective date is available)
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s) A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
Effective Date
ISO Path /MedicinalProduct/ManufacturerEstablishmentOrganisation/Manufacturing-
BusinessOperation/EffectiveDate
FHIR Element Name
start
FHIR Path RegulatedAuthorization.validityPeriod.start (subject reference to relevant
ActivityDefinition)
Example(s):
2017-06-21
2012-12
1.20.8. (Manufacturing business operation) Medicines Regulatory Agency
Organisation
The applicable Medicines regulatory agency, also referred as national competent authority or
supervisory authority, responsible for issuing the manufacturing authorisation should be indicated.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 99/231
Tag Description
User Guidance Medicines regulatory authority shall be specified using the organisation
(ORG ID) and the related location identifier (LOC ID) as listed in the
Organisation Management System (OMS) following a successful
registration of the organisation’s details.
If the required organisation and or related location are not available, the
addition of the unlisted organisation and/or related location should be
requested from OMS using the process described in the OMS Web User
Manual in the 'Documents' section of the Organisation Management
System (OMS).
If the required address of the medicines regulatory authority is not
specified in the documentation, the LOC ID can be left empty.
When the LOC ID is specified, the system would give the information to
which ORG ID the selected location is linked in the back end. This
information will be shown through the PMS User Interface
Repeatable
No
Conformance ORG ID: Mandatory
LOC ID: Conditional (if Manufacturing Authorisation Reference Number is
introduced and the information is available)
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
As listed in SPOR OMS service (ORG ID; LOC ID)
ISO Element Name
MedicinesRegulatoryAgency(Organisation)
ISO Path MedicinalProduct/ManufacturerEstablishmentOrganisation/MedicinesRegula
toryAgency(Organisation)
FHIR Element Name
regulator
FHIR Path RegulatedAuthorization.regulator (subject reference to relevant
ActivityDefinition)

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 100/231
2. Marketing authorisation information
In this section, information about the marketing authorisation shall be specified.
Marketing authorisation is issued by a competent authority, in a specific region, to an organisation that
applied for a marketing authorisation. This is done through a marketing authorisation procedure in
order to place a medicinal product on a market in that specific region. If a marketing authorisation for
a medicinal product is granted, the organisation is referred to as a marketing authorisation holder
(MAH).
The individual national competent authorities (NCA) of the Member States of the European Union (EU)
and the European Economic Area (EEA), grant national marketing authorisation of human medicines
within their territory (i.e., member state). The European Commission grants marketing authorisation to
applications submitted through the centralised procedure, for the authorisation of medicines
throughout the EU. See the Authorisation of medicines webpage for related information.
Where no formal marketing authorisation holder is established, the distributor shall be specified (e.g.,
parallel imported medicinal products submitted on a voluntary basis). However, the obligation to
electronically submit information on all medicinal products for human use, authorised in the European
Union to the EMA, applies to marketing-authorisation holders only. No information needs to be
submitted by parallel distributors in PMS based on the current legal basis and scope of the submission.
The full information on Marketing Authorisation as presented in the FHIR Resource
RegulatedAuthorization is shown in Figure 8 below:
Figure 16: Resource RegulatedAuthorization (source: http://www.hl7.org/fhir)
The Marketing Authorisation class is mandatory and not repeatable while the individual attributes of
this class are not repeatable and shall be populated as applicable.
Marketing authorisation Class Description
Repeatable
No
Conformance
Mandatory

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 101/231
In the context of the Iteration 1 of the PMS implementation, the following elements are in scope and
information should be provided according to the rules and guidance described in this section.
2.1. Regulatory authorisation type
Regulatory applications may be submitted to obtain different types of authorisations or regulatory
entitlements, such as orphan designations, ATMP classification, marketing authorisations etc. in
accordance with the current European regulatory framework for medicinal products. The regulatory
authorisation type “Marketing Authorisation” shall be specified in this section.
In case the marketing authorisation is assigned at the level of the packaged medicinal product, refer to
section 4.7.2. of this Chapter.
Tag Description
User Guidance The type of regulatory authorisation shall be specified when the marketing
authorisation is assigned at the level of medicinal product.
Please note that the RegulatedAuthorization type shall be set as Marketing
Authorisation.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/220000000060
Value(s) As listed in the Regulatory Entitlement Type RMS list.
The RMS term “Marketing Authorisation” shall be selected.
ISO Element Name
Not Applicable
ISO Path
Not Applicable
FHIR Element Name
Type
FHIR Path
RegulatedAuthorization.type
2.2. Marketing authorisation number
Marketing authorisation number or equivalent identifier assigned by the competent authority as
indicated in section 8. Marketing authorisation number(s) of the corresponding SmPC or other
regulatory document(s), shall be specified.
• If the MA number was assigned by the EU Commission, then the MA number as stated in section 8.
Marketing authorisation number(s) of the corresponding SmPC or as stated in the EC decision
document shall be specified.
• If the MA number or equivalent identifier was assigned by the national competent authority and by
EEA countries, then the MA as stated in section 8. Marketing authorisation number(s) of the
corresponding SmPC and applicable NCA’s database or regulated document shall be specified.
• Only one MA number or equivalent identifier can be referenced in this data element.
• Where the authorisation number or equivalent identifier is assigned at Packaged Medicinal Product
level, the stable “root’ number” only shall be provided at the medicinal product level (e.g.,
EU/1/YY/NNNN, applicable to centrally authorized medicinal products). Any authorisation numbers
or equivalent identifier assigned to the individual packages shall be provided at the package level

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 102/231
as described in section 4.7.2. Marketing authorisation number (Package level). This is to avoid
creation of duplication of product entries based of the granularity of authorisation number. This
rule applies to authorised medicinal products with one or more than one presentation.
• Where the authorisation number or equivalent identifier is assigned at Packaged Medicinal Product
level and no stable “root number” common to all packaged medicinal products is assigned, then
this section is to be left blank and it is mandatory to provide the marketing authorisation number
or equivalent identifier at packaged medicinal product level (refer to section 4.7). This rule applies
to authorised medicinal products with one or more than one presentation.
• Where the authorisation number or equivalent identifier is assigned at the level of the Medicinal
Product and the same authorisation number or equivalent identifier applies to more than one
package, the authorisation number or equivalent identifier shall be reported in this section only.
This is to avoid creation of duplication of same product entries based on the number of authorised
Packaged Medicinal Products.
Tag Description
User Guidance Marketing Authorisation number shall be specified.
• Where the authorisation number is assigned at Packaged Medicinal
Product level, the stable “root number” common to all packaged
medicinal products shall be specified.
• Where the authorisation number is assigned at Packaged Medicinal
Product level and no stable “root number” common to all packaged
medicinal products is assigned, this section is to be left blank and it is
mandatory to provide the marketing authorisation number at packaged
medicinal product level (refer to section 4.7.2).
This section is to be completed when the information is available. Because
the Marketing Authorisation Number or equivalent identifier is released by
the competent authority after the end of the regulatory procedure, this
data field can only be populated with the necessary information upon
formal conclusion of the regulatory procedure as this information will not
be available at the time of the submission of the FHIR message (initial
sequence).
Repeatable
No
Conformance
Conditional
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s) The number as assigned by the competent authority of a
country/jurisdiction shall be specified as free text.
The format of the EU number root number shall be "EU/1/YY/NNN " or
"EU/1/YY/NNNN" (as applicable)
ISO Element Name
Marketing Authorisation Number
ISO Path
/MedicinalProduct/MarketingAuthorisation/MarketingAuthorisationNumber
FHIR Element Name
Identifier
FHIR Path
RegulatedAuthorization.identifier
FHIR Complementary
Information
RegulatedAuthorization.identifier.system value is
“http://ema.europa.eu/fhir/marketingAuthorizationNumber”
Example(s):

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 103/231
Where the local national competent authority assigns authorisation numbers as per example below,
where each number is corresponding to a different package with a common root number to all
packages:
Example 1
9743/2016/01-02-03-07
The authorisation number captured at this level should be entered as 9743/2016 whereas
9453/2016/01, 9743/2016/02 etc. will be captured at package level.
Example 2
EU/1/13/016/001
EU/1/13/016/002
EU/1/13/016/003
The authorisation number captured at this level should be entered as EU/1/13/016, whereas the full
MA (EU Number): EU/1/13/016/001, EU/1/13/016/002, EU/1/13/016/003 will be captured at package
level.
2.3. Country
Tag Description
User Guidance The country code of the country where the marketing authorisation was
granted shall be specified as a term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
• For medicinal products authorised via the centralised procedure,
"European Union (EU)" shall be specified.
• For medicinal products authorised in Liechtenstein, Norway and Iceland
via the centralised procedure, the country code (i.e., LI/NO/IS) shall
be specified.
• For medicinal products authorised via national or MRP/DCP procedure,
the EEA country shall be specified.
• For medicinal products authorised outside the EU/EEA area, a non-EEA
country shall be specified.
Medicinal products authorised outside the EU/EEA area may be
optionally submitted. Such medicinal products are not within the scope
of Article 57(2) requirements.
This value is an attribute within Marketing Authorisation domain.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000002
Value(s)
As listed in the Country RMS list

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 104/231
Tag Description
ISO Element Name
Country
ISO Path
/MedicinalProduct/MarketingAuthorisation/Country
FHIR Element Name
Region
FHIR Path
RegulatedAuthorization.region
Example(s):
100000000529 – Kingdom of Spain
100000000390 - European Union
2.4. Authorisation status
Tag Description
User Guidance The status of the marketing authorisation of the medicinal product
throughout its lifecycle shall be specified as a term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
A different authorisation status can apply to each package of the same
medicinal product (e.g., one package can be withdrawn while another is
still valid). The authorisation status of the medicinal product can only be
one.
Therefore, the following rules shall be followed to fill the authorisation
status field at medicinal product level:
• If all the packages have the same authorisation status, this value
should be used at product level.
• If different values apply to the different packages, then, the following
priority list should be used. That means that the first term in this list
used in any package will define the authorisation status at product
level.
Valid > Valid Transferred > Valid Renewed > Valid after lifting of
suspension > Suspended > Expired due to Sunset Clause > Expired >
Withdrawn > Not renewed > Revoked
This value is an attribute within Marketing Authorisation domain.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072049
Value(s)
As listed in the Regulatory Entitlement Status RMS list
ISO Element Name
Authorisation Status
ISO Path
/MedicinalProduct/MarketingAuthorisation/AuthorisationStatus
FHIR Element Name
Status
FHIR Path
RegulatedAuthorization.status
Example(s):

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 105/231
Not renewed
Expired
Revoked
Valid
2.5. Authorisation status date
Tag Description
User Guidance • The date at which the authorisation status has become effective shall
be specified when available.
• The date when the first authorisation was granted by the authorising
body or the date when the renewal was granted (whichever is the
latest) shall be specified in line with section 9. Date of first
authorisation/renewal of the authorisation of the SmPC or any other
regulatory document.
• The date of expiry/revocation/withdrawal of the MA shall be specified if
the MA was:
− withdrawn by the MAH,
− revoked by the competent authority,
− not renewed by the competent authority,
− not submitted for renewal by the MAH,
− expired due to Sunset Clause.
• The date of suspension of the MA shall be entered if the authorisation
status is set to Suspended.
• The date of renewal of the MA shall be entered if the authorisation
status is set to Valid – after renewal
• In case that status “Valid after lifting of suspension is selected” in
previous attribute the date of lifting of the suspension shall be entered.
Repeatable
No
Conformance
Conditional
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s) A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
Authorisation Status Date
ISO Path
/MedicinalProduct/MarketingAuthorisation/AuthorisationStatusDate
FHIR Element Name
statusDate
FHIR Path
RegulatedAuthorization.statusDate
2.6. Date of first authorisation
Tag Description
User Guidance The date when the first authorisation (either from an initial marketing
authorisation application regulatory procedure or line extension application
regulatory procedure) was granted by the competent authority. This

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 106/231
Tag Description
information shall be specified once the medicinal product has been
authorised.
This information shall be in line with the information indicated in section 9.
Date of first authorisation/renewal of the authorisation of the
corresponding SmPC, if this refers to: the first authorisation and not to a
renewal, to other regulatory documents (e.g., European Commission
decision for CAPs) or databases from a National Competent
Authorities/Regulator.
For medicinal products authorised in Liechtenstein, Norway and Iceland
through the centralised procedure the Date of First Authorisation (i.e.,
LI/NO/IS) might be different. In this case, this date is country specific and
therefore, different first approval dates can be reported per
country/region.
In cases of old medicinal products where the first marketing authorisation
was granted in early 1900s and the Date of First Authorisation is unknown
this data element can be populated with the generic value: “Unknown”.
Repeatable
No
Conformance
Mandatory
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s)
A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
When the date is not known the value part of the dateDateTime element
can be missed out (keeping the element itself), and the reason for this
specified using the data absent reason extension, with a value of
“unknown”.
ISO Element Name
Date of First Authorisation
ISO Path
/MedicinalProduct/MarketingAuthorisation/DateOfFirstAuthorisation
FHIR Element Name
dateDateTime
FHIR Path RegulatedAuthorization.relatedDate.dateDateTime or
RegulatedAuthorization.relatedDate.dateDateTime.extension.dataAbsentRe
ason
FHIR Complementary
Information
RegulatedAuthorization.relatedDate.type.system value is
“http://ema.europa.eu/fhir/authorisationDateType”
RegulatedAuthorization.relatedDate.type.code is “dateOfFirstAuthorisation”
Example(s):
The SmPC states the following:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 107/231
Date of first authorisation should therefore reference 2005-10-05. Example(s):
The SmPC states the following:
Date of first authorisation should therefore reference 2004-06-04.
2.7. International birth date
The international birth date (IBD), is defined as the date of the first marketing authorisation for any
medicinal product for human use containing the active substance, granted to any company in any
country in the world.
The European birth date (EBD) is defined as the date of the first marketing authorisation granted by a
decision of the European Commission for a medicinal product for human use to the MAH in the EU.
In the case that the medicinal product containing the active substance is first authorised in European
Union, the European Birth Date equals the IBD.
If the IBD is unknown to the user, the user can refer to the information available under European
Union reference date (EURD) column of the EU reference dates and frequency of submission of PSURs
(EURD list), to populate this data element.
The user should consider that the European Union reference date corresponds to the date of the first or
the earliest known date of the marketing authorisation in the Union of a medicinal product containing
the active substance or combination of active substances [DIR Art 107c (5) (a,b)]. Where the term
“Not available*” is indicated in the list it means that the EURD has not been provided during the
various rounds of consultation on the EURD list with the NCAs and the MAHs. In this case the user
should make his best effort to populate the data element with the relevant and most complete
information.
In cases of old medicinal products where the first marketing authorisation was granted in early 1900s)
the IDB might be unknown and not reported in the EURD list. In such cases the user should include the
value “Unknown”.
Tag Description
User Guidance The date at which the very first marketing authorisation for a new
medicinal product in any country in the world was granted shall be
specified.
This information shall be specified when International Birth Date is
available.
In cases the first authorisation is being obtained in the EU and corresponds
with this product, this information is to be completed once available.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 108/231
Tag Description
If the medicinal product is authorised first in the Europe Union, the
International birth date can be the same of the European Union reference
date.
If the medicinal product is authorised first in country other than the
European Union, the International birth date does not correspond to the
European Birth Date, however in some cases it might still be found in the
EURD list.
• In case of very old medicinal products, where the IBD is unknown
(therefore is not available in the EURD list) the IBD can be populated
with the generic value: “Unknown”.
In case of medicinal product with no active ingredient, the IBD can be left
blank
Repeatable
No
Conformance Mandatory
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s)
A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
When the date is not known the value part of the dateDateTime element
can be missed out (keeping the element itself), and the reason for this
specified using the data absent reason extension, with a value of
“unknown”.
ISO Element Name
International Birth Date
ISO Path
/MedicinalProduct/MarketingAuthorisation/InternationalBirthDate
FHIR Element Name
dateDateTime
FHIR Path RegulatedAuthorization.relatedDate.dateDateTime or
RegulatedAuthorization.relatedDate.dateDateTime.extension.dataAbsentRe
ason
FHIR Complementary
Information
RegulatedAuthorization.relatedDate.type.system value is
“http://ema.europa.eu/fhir/authorisationDateType”
RegulatedAuthorization.relatedDate.type.code is “internationalBirthDate”
Example(s): Medicinal product authorised first time in the world in country other than European
Union.
The SmPC of the medicinal product “Accofil” states the following:
The relevant EC European Commission decision states the following:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 109/231
Date of first authorisation should therefore reference 2014-09-18.
In the EURD list, at the corresponding section of the active ingredient “filgrastim” contained in the
medicinal product above reported states the following:
International birth date should therefore reference 1991-03-15.
Example(s): Medicinal product authorised first time in the world in country other than European
Union.
The SmPC of the medicinal product “Vaxzevria” states the following:
The relevant EC European Commission decision states the following:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 110/231
Date of first authorisation should therefore reference 2021-01-29.
In the EURD list, at the corresponding section of the active ingredient “COVID-19 Vaccine (ChAdOx1-S
[recombinant])” (COVID-19 Vaccine AstraZeneca) contained in the medicinal product above reported
states the following:
International birth date should therefore reference 2020-12-29.
Example(s): Medicinal product authorised first time in the world in European Union.
The SmPC of the medicinal product “Zynteglo” states the following:
The relevant EC European Commission decision states the following:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 111/231
Date of first authorisation should therefore reference 2019-05-29.
In the EURD list, at the corresponding section of the active ingredient “betibeglogene autotemcel”
contained in the medicinal product above reported states the following:
International birth date should therefore reference 2019-05-29.
2.8. Marketing authorisation holder (organisation)
The information on the holder of the marketing authorisation (RegulatedAuthorization.holder) of the
medicinal product, in each country, as indicated in section 7. Marketing Authorisation Holder of the
SmPC or other regulatory document (e.g., European Commission Decision for CAPs) shall be specified
as an Identifier and following a successful registration of organisation's information into the
Organisation Management System (OMS)
.
Parallel distributed/imported medicinal products which may be distributed without a marketing
authorisation under regional law [Article 76(3) and (4) of Directive No2001/83/EC], and are out of
scope or Article57 submission, may be provided to the EMA on a voluntary basis. The details of the
distributor, as specified on the package, the container or the package insert shall be provided in place
of the MAH details.
Tag Description
User Guidance The Marketing authorisation holder shall be specified using the location
identifier (LOC ID) linked to the organisation as listed in the Organisation

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 112/231
Tag Description
Management System (OMS) following a successful registration of the
organisation’s details.
If the required organisation and/or related location are not available, the
addition of the organisation and/or related location should be requested
from OMS, using the process described in the OMS Web User Manual
available in the 'Documents' section of the Organisation Management
System (OMS).
When the LOC ID is specified, the system would give the information to
which ORG ID the selected location is linked in the back end. This
information will be shown through the PMS User Interface
Repeatable
No
Conformance
Mandatory
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
As listed in SPOR OMS (LOC ID)
ISO Element Name
MarketingAuthorisationHolder(Organisation)
ISO Path MedicinalProduct/MarketingAuthorisation/MarketingAuthorisationHolder(Or
ganisation)
FHIR Element Name
Authorisation.holder
FHIR Path
RegulatedAuthorization.holder
2.9. (Marketing authorisation) Regulator
The regulator (RegulatedAuthorization.regulator) shall be specified as an Identifier and following a
successful registration of organisation's information into the Organisation Management System (OMS).
Tag Description
User Guidance Medicines regulatory authority shall be specified using the organisation
identifier (ORG ID) and the related location identifier (LOC ID) as listed in
the Organisation Management System (OMS) following a successful
registration of the organisation’s details.
If the required organisation and/or related location are not available, the
addition of the organisation and/or related location should be requested
from OMS using the process described in the OMS Web User Manual
available in the 'Documents' section of the Organisation Management
System (OMS).
If the required address of the medicines regulatory authority is not
specified in the documentation, the LOC ID can be left empty.
When the LOC ID is specified, the system would give the information to
which ORG ID the selected location is linked in the back end. This
information will be shown through the PMS User Interface.
Repeatable
No
Conformance ORG ID: Mandatory
LOC ID: Conditional
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
As listed in SPOR OMS (ORG ID; LOC ID)

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 113/231
Tag Description
ISO Element Name
Identifier
ISO Path MedicinalProduct/MarketingAuthorisation/Organisation
(MedicinesRegulatoryAgency)/Identifier
FHIR Element Name
Regulator
FHIR Path
RegulatedAuthorization.regulator
2.10. Marketing authorisation procedure
Marketing Authorisation Procedure class is used for submitting information related to Marketing
authorisation approval routes (e.g., centralised procedure, mutual recognition procedure, decentralised
procedure and national Procedure) and regulatory procedure applications (e.g., initial marketing
authorisation application, variations, transfers, periodic safety update reports etc..), that impact the
product information as included in this guidance.
Figure 17: Extract of the Marketing authorization procedure class (source: http://www.hl7.org/fhir
The class is mandatory and repeatable for medicinal products authorised in the EU/EEA.
This class is optional and repeatable for medicinal products authorised outside the EU/EEA and
submitted on a voluntary basis.
The individual attributes of this class are not repeatable and shall be populated as applicable.
Marketing authorisation procedure Class Description
Repeatable No (medicinal products authorised in/outside
EU /EEA)
Conformance Mandatory (medicinal products authorised in
EU/EEA)
Optional (medicinal products authorised
outside EU/EEA)
2.10.1. Procedure Identifier
The procedure number assigned by the competent authority to a specific authorisation procedure shall
be specified.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 114/231
Procedure Identifier relates exclusively to the initial authorisation procedure registration route and
should be completed once. In addition, in case of a switch from a purely National Procedure to Mutual
recognition procedure (MRP) this attribute shall be completed again (updated).
• Mutual recognition procedure (MRP) number or Repeat Use Procedure (RUP) shall be specified
when the authorisation procedure is entered as 'Mutual Recognition Procedure'. The format of the
MRP number:
− shall be the same as Module 1.2 - Electronic Application Form or as stated in the MR Index on
the Heads of Medicines Agency's website
(without the reference to the marketing application,
i.e., the text "/MR" or "E")
Information on application number referring to initial marketing authorisation or line extension is
not to be reflected in the procedure number (no addition behind the procedure number in bold
CC/D/nnnn/sss/QQ/vvv, therefore no QQ/vvv part added, e.g., IE/H/0234/001). The format as
reflected in
CMDh Guidance Document on the Numbering System for the Procedures for Mutual
Recognition and Decentralised Procedure should be used.
• Decentralised authorisation procedure (DCP) number shall be specified when the authorisation
procedure is entered as Decentralised Procedure. The format of the DCP number should be as
stated in in Module 1.2 - Electronic Application Form or the MR Index on the
Heads of Medicines
Agency's website, i.e., without the reference to the marketing application (if included, without the
reference to the marketing application, i.e., the text "/DC") . EMA “EMEA procedure number” (i.e.,
"Agency Product Number" as referred to/published on the
EMA corporate website shall be specified
when the authorisation procedure is entered as Centralised Procedure.
The format of the EMEA procedure number shall be EMEA six-digit procedure number (i.e.,
EMEA/H/C/123456).
• For purely national authorisation procedures, the local procedure number or national identifier shall
be provided.
• For procedures carried outside EU, the relevant procedure identifier may be included (on a
voluntary basis) when entering a medicinal product authorised outside the EEA.
Tag Description
User Guidance Procedure number should be specified in accordance with information in
Module 1.2 - Electronic Application Form.
This section is to be completed when the information is available.
Repeatable
No
Conformance
Conditional
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
The applicable procedure number shall be specified as free text.
ISO Element Name
Procedure Identifier / Number
ISO Path /MedicinalProduct/MarketingAuthorisation/MarketingAuthorisationProcedur
e/ProcedureIdentifier-Number
FHIR Element Name
Identifier
FHIR Path
RegulatedAuthorization.case.identifier

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 115/231
Tag Description
FHIR Complementary
Information
RegulatedAuthorization.case.identifier.system value is
“http://ema.europa.eu/fhir/procedureIdentifierNumber”
Example(s):
SE/H/1111/222
DE/H/1111/001
EMEA/H/C/123456
2.10.2. Procedure type – Medicines approval system
The type of procedure (EU medicinal marketing authorisation approval routes) corresponds with the
medicines approval system through which the initial marketing authorisation application is being
assessed or was granted (for already approved products) by the regulatory authority. This information
shall be specified.
• Centralised procedure
is to be specified when entering a centrally authorised medicinal product.
− The authorisation country code shall have been specified as ’EU' in section 2.3 “Country”;
− For medicinal products authorised in Liechtenstein, Norway and Iceland via the centralised
procedure, the applicable country (i.e., LI/NO/IS) shall have been specified in section 2.3
“Country”
• Mutual recognition procedure
is to be specified when entering a mutually recognised medicinal
product and in the of case of a repeat-use procedure.
− The authorisation country shall have been specified as one of the EEA countries in section 2.3
“Country”.
• National procedure
is to be specified when entering a nationally authorised medicinal product.
− The authorisation country shall have been specified as one of the EEA countries in section 2.3
“Country”.
• Decentralised procedure
is to be selected when entering a medicinal product authorised via a
decentralised procedure:
− The authorisation country shall have been specified as one of the EEA countries in section 2.3
“Country”.
• Non-EU Authorisation Procedure is to be selected when a medicinal product is authorised via a
procedure carried outside EU and when entering a medicinal product authorised outside the EEA
(on a voluntary basis).
− The authorisation country code shall not have been specified as "EU" or any of the EEA
countries in section 2.3 “Country”.
Tag Description
User Guidance The type of procedure (EU medicinal marketing authorisation approval
routes) through which the initial marketing authorisation was granted by
the regulatory authority shall be specified.
The value(s) shall be selected from the term ID as listed in the
Referentials Management Service (RMS) list.
Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 116/231
Tag Description
NOTE: The relevant RMS list contains terms which can be used for
medicinal products that can be submitted into PMS in a voluntary basis.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000154442
Value(s) As listed in the EU Regulatory Authorisation/Registration Procedure RMS
list
ISO Element Name
Procedure Type
ISO Path /MedicinalProduct/MarketingAuthorisation/MarketingAuthorisationProcedur
e/ProcedureType
FHIR Element Name
Type
FHIR Path
RegulatedAuthorization.case.type
Example(s):
Centralised Procedure
Decentralised Procedure
Mutual Recognition Procedure
National Procedure
Non-EU authorisation procedure
2.10.3. Procedure start date
Tag Description
User Guidance The date when the marketing authorisation procedure started may be
specified when available.
Repeatable
No
Conformance
Conditional
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s)
A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
Procedure Date Start
ISO Path /MedicinalProduct/MarketingAuthorisation/MarketingAuthorisationProcedur
e/ProcedureDateStart
FHIR Element Name
Start
FHIR Path
RegulatedAuthorization.case.datePeriod.start

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 117/231
2.10.4. Procedure end date
Tag Description
User Guidance The date when the marketing authorisation procedure was completed shall
be specified when available.
• For centralised procedure, this date corresponds with the date of
European Commission (EC) decision of the initial marketing
authorisation. If the medicinal product was registered through a line
extension, the date of European Commission (EC) decision of the
related product with a different strength/pharmaceutical form shall be
selected.
• For mutual recognition procedure, this date corresponds with the date
of each national competent authority decision of the initial marketing
authorisation (date when the MA is granted).
• For decentralised procedure, this date corresponds with the date of
each national competent authority decision of the initial marketing
authorisation (date when the MA is granted).
• For national procedures, this date corresponds with the date of
national competent authority decision of the initial marketing
authorisation (date when the MA is granted).
Repeatable
No
Conformance
Conditional
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s) A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
Procedure Date End
ISO Path /MedicinalProduct/MarketingAuthorisation/MarketingAuthorisationProcedur
e/ProcedureDateEnd
FHIR Element Name
End
FHIR Path
RegulatedAuthorization.case.datePeriod.end
2.10.5. Regulatory application
A marketing authorisation and associated lifecycle activities are linked to regulatory procedures. These
include the initial marketing authorisation application and subsequent applications for changes to the
existing marketing authorisation (e.g., line extensions, renewals, variations), that occur during the
lifecycle of the medicinal product).
The initial regulatory procedure application leading to the registration of the medicinal product for the
first time (e.g., initial marketing authorisation, line extension if other strengths/pharmaceutical
products of the same global marketing authorisation) shall be recorded.
If applicable, details of the marketing authorisation application related to the subsequent changes of
the marketing authorisation should be captured as described in the following section. It is important to
note that in FHIR both procedures and applications are modelled as cases, and that there is a recursive

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 118/231
relationship between cases (a case can be a parent of other cases). It is expected that applications
that belong to a procedure are expressed as elements of the procedure (children).
The regulatory application class and the individual attributes of this class are conditional (except for
the regulatory application type data field) and not repeatable
Regulatory application Class Description
Repeatable
No
Conformance
Conditional
2.10.5.1. Regulatory application Identifier/Number
Tag Description
User Guidance The number assigned to the marketing authorisation application (including
post-authorisation procedure applications such as variations, renewals and
other regulatory procedure applications) by the regulatory agency shall be
described in text.
Note 1: In some member states a regulatory applications
identifier/number is not assigned for medicinal products approved through
national procedure route. In these cases, this field should be left blank.
This section is to be completed when the information is available.
Note 2: only the regulatory application procedures affecting attributes
included in the PMS data model will trigger the need to update this section.
Regulatory application procedures information not affecting attributes in
the PMS data model do not need to be recorded.
Repeatable
No
Conformance
Conditional
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
The applicable procedure number shall be specified as free text.
ISO Element Name
Application Identifier / Number
ISO Path /MedicinalProduct/MarketingAuthorisation/MarketingAuthorisationProcedur
e/MarketingAuthorisationApplication/ApplicationIdentifier-Number
FHIR Element Name
Identifier
FHIR Path
RegulatedAuthorization.case.application.identifier
FHIR Complementary
Information
RegulatedAuthorization.case.application.identifier.system value is
“http://ema.europa.eu/fhir/applicationIdentifierNumber”
Example(s):
EMEA/H/C/123456/X/001
EMEA/H/C/123789/000
EMEA/H/C/123789/II/003
DE/H/1111/001/II/001
EE/H/0220/003/IB/012

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 119/231
DE/H/0210/001/WS/136
2.10.5.2. Regulatory application type
Tag Description
User Guidance The type of regulatory application shall be described using a term ID.
• The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
• In case of grouping of variations, the application submission type with
the highest ranking of variation shall be selected.
This value is an attribute within the Regulatory application procedure
Identifier/Number.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL https://spor.ema.europa.eu/v1/lists/100000155688
Value(s)
As listed in the Application Submission Type RMS list
ISO Element Name
Application Type
ISO Path /MedicinalProduct/MarketingAuthorisation/MarketingAuthorisationProcedur
e/MarketingAuthorisationApplication/ApplicationType
FHIR Element Name
Type
FHIR Path
RegulatedAuthorization.case.application.type
Example(s):
Initial Marketing Authorisation Application
(100000155689)
Renewal (100000155697)
Variation Type IB (100000155692)
Variation Type II (100000155693)
2.10.5.3. Regulatory application end date
Tag Description
User Guidance The date when the regulatory application procedure was completed (if
available) shall be specified, when available.
• For centralised procedure, this date corresponds with:
− date of European Commission (EC) decision: procedures that
requires of EC decision;
− date of finalisation of the linguistic review: procedures without EC
decision and where a linguistic review of the Product information is
needed;
− date of the regulatory procedure opinion in cases where no
linguistic review and EC decision is required.
• For mutual recognition and decentralised procedure, this date
corresponds with:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 120/231
Tag Description
− the regulatory application end date is not what is usually called the
End of Procedure in the European phase of the MRP/DCP approval
process for applications for new products, extensions or variations.
It corresponds to the date of each national competent authority
decision: procedures that requires of NCA decision;
−
− date of finalisation of the linguistic review: procedures without NCA
decision and where a linguistic review of the Product information is
needed;
− date of the regulatory procedure opinion in cases where no
linguistic review and NCA decision is required.
• Fore pure national procedures, the applicable date is dependent on
the national procedures.
Repeatable
No
Conformance
Conditional
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s) A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e.,, YYYY-MM).
ISO Element Name
Application Date
ISO Path /MedicinalProduct/MarketingAuthorisation/MarketingAuthorisationProcedur
e/MarketingAuthorisationApplication/ApplicationDate
FHIR Element Name
dateDateTime
FHIR Path
RegulatedAuthorization.case.application.dateDateTime

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 121/231
3. Therapeutic (product) indication
Therapeutic (product) indications will be implemented using the FHIR ClinicalUseIssue Resource (see
Figure 9). In the context of the Iteration 1 of the PMS implementation, the elements in red rectangle
are in scope and shall be provided according to the rules and guidance described in this section.
Figure 18: Resource ClinicalUseIssue (source : http://www.hl7.org/fhir
)
The Therapeutic (product) indication class is mandatory and repeatable while the different attributes
shall be populated as applicable and are not repeatable (except for the Co-morbidity data element).
Therapeutic (product) indication Class Description
Repeatable
Yes
Conformance
Mandatory
3.1. Indication as "Disease/Symptom/Procedure"
10
Tag Description
User Guidance The coding of the authorised indication(s) as disease, symptom or
procedure as reflected in Section 4.1 Therapeutic Indications of the
corresponding SmPC or other regulatory document shall be specified as an
RMS term ID.
• The applicable Lowest Level Terms (LLT) from the Medical Dictionary
for Regulatory Activities (MedDRA) value(s) shall be selected from the
10
Description of the authorised full therapeutic indication(s) as text, as reflected in Section 4.1 Therapeutic
Indications of the corresponding SmPC or other regulatory document, has been described in section
1.11.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 122/231
Tag Description
term ID as available in applicable Referentials Management Service
(RMS) list. The selected term will be shown only in English language
only. This differ from the possibility to have the information in multiple
languages in the 1.11 Full indication text data element.
• The chosen MedDRA term should be as specific as possible, targeting
to capture the most detailed level of information presented in the
indication section.
Note: To achieve a greater specificity of the coded term, it is
recommended to select the MedDRA term that accounts for as much
information as possible, such as:
• Both the disease and its cause as provided in the SmPC, (e.g.,
‘Osteoporosis steroid -induced’);
• Aspects of ‘Disease status specification’ (e.g., Pancreatic
adenocarcinoma metastatic);
• Details related to the target population (e.g., ‘Osteoporosis
postmenopausal)
•
Timing/duration (e.g., ‘Hepatitis chronic persistent’)
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000006
Value(s) As listed in the RMS list containing the terms from the Medical Dictionary
For Regulatory Activities list (MedDRA)
ISO Element Name
Indication as "Disease / Symptom / Procedure"
ISO Path /MedicinalProduct/TherapeuticIndication/IndicationAsDisease-Symptom-
Procedure
FHIR Element Name
diseaseSymptomProcedure
FHIR Path
ClinicalUseIssue.indication.diseaseSymptomProcedure
Example(s):
100000054740 Migraine headache; 100000006570 Vascular hypotensive disorders; 100000034529
Pain relief
3.2. Co-morbidity
Tag Description
User Guidance The description of any comorbidity [i.e., concurrent condition(s)] or co-
infections included in the authorised indication(s) as reflected in Section
4.1 Therapeutic Indications of the SmPC may be specified using the RMS
term ID.
This refers to further conditions (concurrent conditions or co-infections)
that define the patient population apart from the
‘Disease/symptoms/procedure’ aspect of the indication.
• The applicable Lowest Level Terms (LLT) from the Medical Dictionary
for Regulatory Activities (MedDRA) value(s) shall be selected from the
term ID as available in applicable Referentials Management Service

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 123/231
Tag Description
(RMS) list. The selected term will be shown only in English language
only. This differ from the possibility to have the information in multiple
languages in the 1.11 Full indication text data element.
• The chosen MedDRA term should be as specific as possible, targeting
to capture the most detailed level of information presented in the
indication section.
Repeatable
Yes
Conformance
Conditional
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000006
Value(s)
As listed in the Medical Dictionary For Regulatory Activities RMS list
ISO Element Name
Comorbidity
ISO Path
/MedicinalProduct/Contraindication/Comorbidity
FHIR Element Name
comorbidity
FHIR Path
ClinicalUseIssue.indication.comorbidity
To exemplify the separation between ‘Indication as Disease/Symptom/Procedure’ and
‘Comorbidity/concurrent disease’, where the indication text states: “Treatment of pancreatic
insufficiency in patients with cystic fibrosis”, then ‘pancreatic insufficiency’ needs to be included as
indication, whereas ‘cystic fibrosis’ represents the ‘Comorbidity/concurrent conditions’ aspect of the
indication and it should not be included at this stage in the indication field.
Careful consideration of the drug’s mechanism of action and impact on the disease is required when
assessing whether the diseases mentioned in the indication text are considered to be ‘comorbidity’ or
whether they constitute the therapeutic indication (and need to be included). Treatment of typical
signs and symptoms of a disease may represent treatment of the disease.
Looking at an example where indication states: “For the treatment of hyperglycaemia in type II
diabetes”, then it needs to be considered that treatment of hyperglycaemia (i.e., achieving
normoglycaemia), is the goal of all antidiabetic treatment, and type II diabetes should therefore be
considered as the indication for treatment and not a concurrent disease.
Example(s):
Figure 19: section 4.1 SmPC, Indication as disease/symptom/procedure, Intended effect,
Comorbidity/concurrent disease
As general principle, one ClinicalUseIssue resource shall be created per each of the listed therapeutic
indications. In the example reported in figure 10 there are three therapeutic indications, one intended
effect and one comorbidity, therefore three ClinicalUseIssue resource shall be created:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 124/231
• TI (Increased urinary frequency) + Intended effect (Treatment) + Comorbidity (Overactive
bladder);
• TI (Urge incontinence) + Intended effect (Treatment) + Comorbidity (Overactive bladder)
• TI (Urinary urgency) + Intended effect (Treatment) + Comorbidity (Overactive bladder)
An example where both indications as captured in SmPC are to be coded:
Figure 20: Coding both indications as captured in SmPC
An example where most detailed information is captured, capturing also the context of the symptom
(comorbidity/concurrent disease).
Figure 21: Detailed information as captured together with the context of the symptom
(comorbidity/concurrent disease)
3.3. Intended effect
Tag Description
User Guidance The intended effect (i.e., the part of the indication that describes the
result/type of outcome intended for the target condition), aim or strategy
to be achieved by the indication as reflected in Section 4.1 Therapeutic
Indications of the corresponding SmPC or other regulatory document shall
be specified using an RMS term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Note: Special attention should be given to situations where a drug is
indicated also for treatment and not only for prevention. If a medicinal
product is also authorised for the treatment of a disease, then the
respective disease should also be coded.
Repeatable
Yes

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 125/231
Tag Description
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/rmswi/#/lists/200000003186
Value(s) As listed in the Medicine Profile RMS List
This RMS list is hierarchical. Users can only see and select the first level of
terms to applies to PMS for data entry purposes.
ISO Element Name
Intended Effect
ISO Path
/MedicinalProduct/TherapeuticIndication/IntendedEffect
FHIR Element Name
intendedEffect
FHIR Path
ClinicalUseIssue.indication.intendedEffect
Example(s):
Treatment, Prophylaxis, Diagnosis
Figure 22 shows an example where the indication needs to be carefully considered in its context
(migraine is not a concurrent condition in this case - it represents the disease to be treated). Also
shows how the intended effect should be captured:
Figure 22: Example where indication needs to be carefully considered in its context
Figure 23 shows an example where both treatment and prophylaxis are to be coded in indication
section, as the medicinal product is used for both purposes
Figure 23: Treatment and prophylaxis coded in indication section
Figure 24 shows an example where the capturing of indication of a replacement therapy is presented:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 126/231
Figure 24: Capturing of indication of a replacement therapy

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 127/231
4. Packaged medicinal product
This section describes the packaging/container(s) information of a medicinal product and any
associated device(s) which are an integral part or provided in combination with a medicinal product, as
supplied for sale or distribution/supply. This section should be completed as follows:
• a Medicinal Product shall be associated to one or more Packaged Medicinal Products;
• a Packaged Medicinal Product is associated with the PCID.
The package of the medicinal product shall be described from the outer to inner part of the medicinal
product and down to the individual ingredient(s) of each packaged pharmaceutical form. For this
purpose, the following classes apply:
• The Packaged Medicinal Product class: overarching class
− The Package Item (Container) class [i.e., the outer and inner primary and secondary
package(s)]
− The Manufactured item/ingredient(s) class [i.e., the individual pharmaceutical
form(s)/ingredient(s)]
− Device class (i.e., administration device co-packaged with the medicinal product or when
medicinal product is integrated within a device such as pre-filled syringes)
− Shelf-Life / Storage Conditions associated to the Packaged Medicinal Product.
The Packaged Medicinal Product class is the overarching class which collects all information on the
individual package within the medicinal product i.e., the ‘Package Item (Container)’ class for each
separate item packaged.
The Package Item (Container) class allows for a recursive relationship, which is required to be used
when there are packages within packages, e.g., cartridges within a blister sleeve within a box. A
Package Item (Container) has the following characteristics:
• It may have 'Package (Component)' parts such as closures.
• A Package Item (Container) can be identified by one or more Data Carrier Identifiers (e.g., Global
Trade Item Number
TM
(GTIN
TM
), National Trade Item Number (NTIN).
Manufactured Item is defined by individual Ingredients and Physical Characteristics and is linked to
specific package item containers.
In the context of implementation of PMS iteration 1, the Packaged Medicinal Product can be
accompanied by a Device (class 'Device').
In addition, the Packaged Medicinal Product will have associated a Shelf-Life / Storage Conditions as
this is associated to the Package Item Container.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 128/231
Example(s):
Packaged Medicinal Product
PCID
EU-100000396-00020080-0001
Package Description
Pack of one vial (type I glass) of 2.5 ml suspension with a
stopper (butyl rubber) and one vial (type I glass) of 2.5ml
emulsion with a stopper (butyl rubber)
Package Item Container (1)
Box
Package Item Container Quantity
1
Package Item Material
Recycled Cardboard
Packaged Item Reference
Reference to Package item 2 and Package item 3
Manufacturing item Reference
Not Applicable
Data Carrier Identifier (s)
02890138016090
Package Item Container (2)
Vial
Package Item Container Quantity
1
Package Item Material
Type I glass
Package Item Component Type
Stopper
Package Item Component
Material
Butyl Rubber
Manufactured Item
(Manufactured Dose Form)
Suspension for emulsion for injection
Packaged Item Reference
Not applicable
Manufacturing item Reference
Reference to Suspension for Emulsion for Injection
Package Item Container (3)
Vial
Package Item Container Quantity
1
Package Item Material
Type I glass
Package Item Component Type
Stopper
Package Item Component
Material
Butyl Rubber
Manufactured Item
(Manufactured Dose Form)
Emulsion for emulsion for injection
Packaged Item Reference
Not applicable
Manufacturing item Reference
Reference to Emulsion for Emulsion for Injection
The descriptions of the attributes and the relevant business rules are described in the sections below.
The example above is fictitious.
The full information on Packaged Medicinal Product, as described by ISO 11615, is implemented based
on the HL7 FHIR Resource PackagedProductDefinition, as shown in Figure 16
.
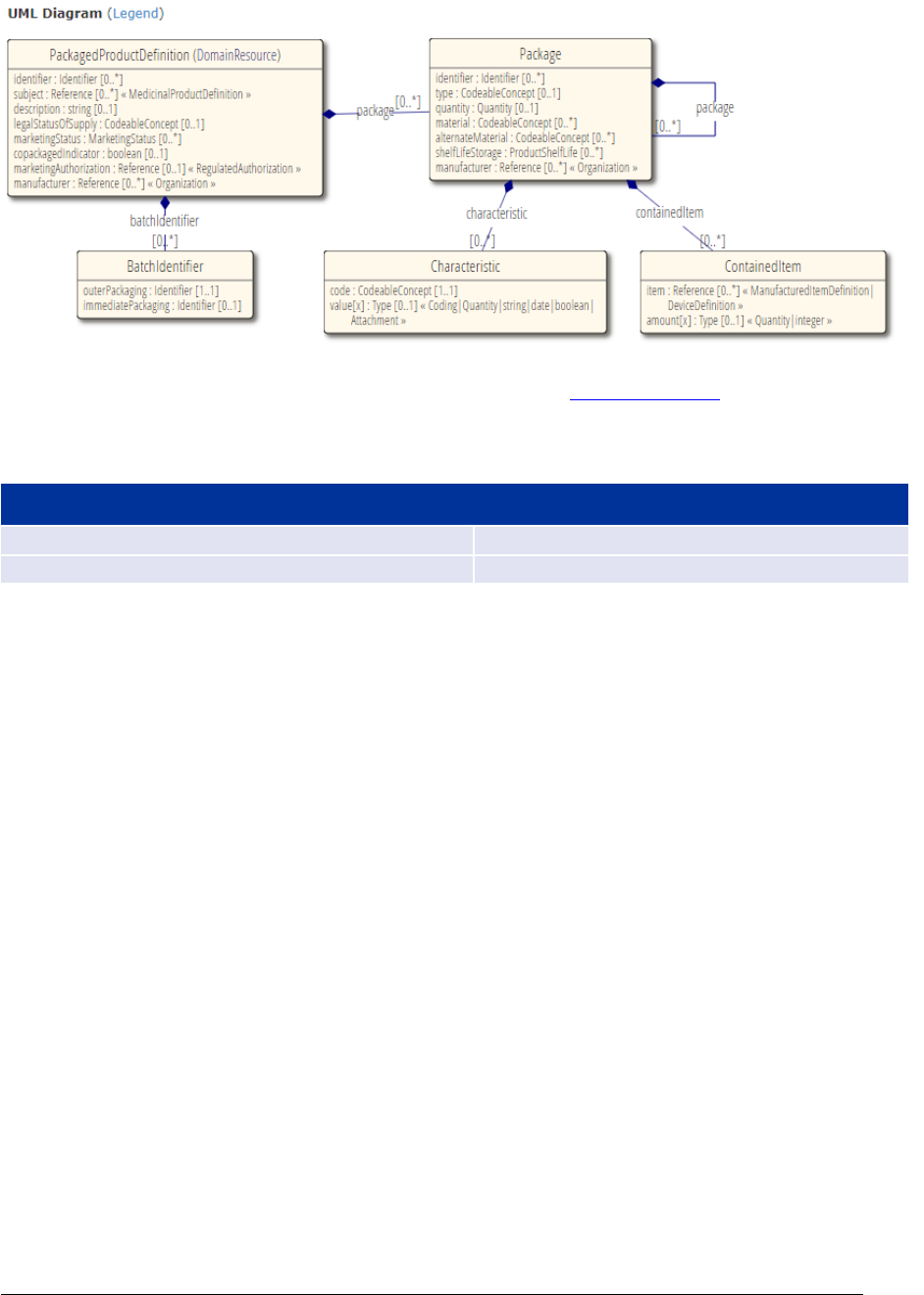
Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 129/231
In the context of the Iteration 1 of the PMS implementation, the following elements are in scope and
information shall be provided according to the rules and guidance described in this section:
Figure 25: FHIR Resource: PackagedProductDefinition (Source: www.hl7.org/fhir
)
The Packaged Medicinal Product class is a mandatory and repeatable while the individual attributes of
this class are repeatable and shall be populated as applicable.
Packaged medicinal product Class Description
Repeatable
Yes
Conformance
Mandatory
Note: The contents of the document [i.e., Module 1.2 – Electronic Application form (eAF), Relevant
sections in Module 3 – Quality, Summary of Product Characteristics (SmPC)] supporting the regulatory
process shall be aligned, where applicable, to ensure the discrepancies between the documents are
minimized. The content should enhance the quality of the product data reported in Product
Management Service (PMS). This requirement applies to new medicinal products single entry in PMS.
Based on the above principle, the SmPC as authorised/to be authorised is the main referring document
for data entry purposes.
However, for medicinal product entry already available in PMS, following the data load from XEVMPD to
PMS database (existing product data), whenever the common contents of each of the above supporting
documentation are not aligned, the information available in the relevant sections in Module 3 can be
used to harmonize the values in PMS. This requirement applies provided data confidentiality is ensured
and if no additional complexity is added to the data entry in PMS. For additional information, refer to
section 1.3.1 of EU IG Chapter 8 – Practical example.
Note: Further information referring to existing product data will be made available in the EU IG
Chapter 9 - Process for submitting existing data on medicinal products authorised for human use. This
chapter is under development and it will be made available at later stage.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 130/231
4.1. Packaged Medicinal Product Identifier (PCID
11
)
Tag Description
User Guidance For each Packaged Medicinal Product, a unique PCID is assigned by the
PMS system based on the data submitted.
It is supplementary to any existing authorisation/approval number at
package level assigned by the Commission or national competent
authorities. There are two components of a PCID:
- MPID for the Medicinal Product;
- Package description code segment, which refers to a unique
identifier for each package e.g., 0001, 0002 etc.
Note: For authorisations which cover only one pack, one PCID will be
assigned, with 0001 as the package description code segment. For
authorisations which cover more than one pack, a PCID will be assigned to
each pack.
Any change of the values related to these code segments shall result in the
assignment of a new PCID.
A unique PCID is assigned according to the following defining attributes of
the package code segment:
- package item (container)(s) — the type, quantity (items per
package), material(s);
- package component(s) — type, material(s)
- manufactured item(s) — manufactured dose form, unit of
presentation, quantity (items per package).
When the above defining attributes sets differ in any way a new PCID will
be assigned by the system
Repeatable
No
Conformance For first data submission and data maintenance: Not applicable – ID
generated by the system.
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
ID generated by the system
ISO Element Name
PCID
ISO Path
/MedicinalProduct/PackagedMedicinalProduct/PCID
FHIR Element Name
Identifier
FHIR Path
PackagedProductDefinition.identifier
FHIR Complementary
Information
PackagedProductDefinition.identifier.system value is
“http://ema.europa.eu/fhir/pcId”
Example(s):
SE-100001745-00040001-0001
EU-100000396-00020080-0001
11
Concept of PCID - © CEN, reproduced with permission
Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 131/231
4.2. Package description
Tag Description
User Guidance A description of the packaged medicinal product in relation to the pack
size(s) in line with the information indicated in section 6.5 Nature and
contents of container of the corresponding SmPC or other regulatory
document shall be specified as text.
Products authorised through MRP/DCP/NP routes
• The package description is to be provided in English or in the local
language(s) of authorisation, or optionally in all of them.
Products authorised through the centralised procedure
•
The package description is to be provided in English
Repeatable
Yes
Conformance
Mandatory
Data Type
Markdown
RMS URI/URL
Not applicable
Value(s) The description of the packaged medicinal product shall be provided as
text.
ISO Element ID
Package description
ISO Path
/MedicinalProduct/PackagedMedicinalProduct/PackageDescription
FHIR Element ID
Description
FHIR Path
PackagedProductDefinition.description
Example(s):
Section 6.5 Nature and contents of container:
84 or 100 tablets in an amber glass bottle.
Information to be entered in Package description of first package:
84 tablets in an amber glass bottle.
Information to be entered in Package description of second package:
100 tablets in an amber glass bottle.
Section 6.5 Nature and contents of container:
Confezione da 14, 28 o 98 compresse rivestite con film in blister
Information to be entered in Package description of PCID1:
Confezione da 14 compresse rivestite con film in blister
Information to be entered in Package description of PCID2:
Confezione da 28 compresse rivestite con film in blister
Information to be entered in Package description of PCID3:
Confezione da 98 compresse rivestite con film in blister

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 132/231
Section 6.5 Nature and contents of container:
Ampoule en verre neutre de 1 ml. 5 ampoules dans un carton.
Information to be entered in Package description of PCID1:
Ampoule en verre neutre de 1 ml. 5 ampoules dans un carton.
4.2.1. Language
This section described how to populate information related to the language of the package description.
The provision of the language is mandatory.
Tag Description
User Guidance The language of the package description as specified in previous section
shall be specified.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072057
Value(s)
As listed in the Language RMS list
ISO Element Name
Not Applicable
ISO Path
Not Applicable
FHIR Element Name
valueCode
FHIR Path PackagedProductDefinition.description.extension.language
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.3. Manufacturer (New)
Tag Description
User Guidance
The reference to the relevant Manufacturer of the packaged medicinal
product shall be selected from the list of manufacturers recorded in section
1.20 Manufacturing business operation.
Repeatable
Yes
Conformance
Conditional
Data Type
Reference
RMS URI/URL
Not applicable
Value(s) Reference to the relevant ActivityDefinition resource describing the
manufacturing business operation.
ISO Element Name
Manufacturer(Organisation)
ISO Path MedicinalProduct/PackagedMedicinalProduct/ManufacturerEstablishmentOr
ganisation/Manufacturer(Organisation)
FHIR Element Name
manufacturer
FHIR Path PackagedProductDefinition.manufacturer.extension.manufacturingBusiness
Operation

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 133/231
Tag Description
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.4. Pack size
Tag Description
User Guidance For each Packaged Medicinal Product, the pack size defined as the total
number of units of the manufactured item or package item and
represented per unit of presentation shall be provided.
For medicinal products with multiple pharmaceutical products (e.g.,
tablet and cream) the pack size shall be differentiated and repeated by
manufactured item/package item.
Example 1: 28 tablets and 1 tube (cream) – In this case the pack size field
is repeated including quantity and unit of presentation for tablets and
quantity and unit of presentation (tube) for the cream.
For medicinal products in solid dosage forms with multiple
pharmaceutical products that present the same unit of
presentation (e.g., contraceptive tablets of different colours and
formulation), the pack size shall be accounted as the total number of
tablets.
Example 2: 28 film coated tablets containing:
2 dark yellow tablets each containing 3 mg estradiol valerate
5 medium red tablets each containing 2 mg estradiol valerate and 2 mg
dienogest
17 light yellow tablets each containing 2 mg estradiol valerate and 3 mg
dienogest
2 dark red tablets each containing 1 mg estradiol valerate
2 white tablets do not contain active substance
In this case the pack size field is completed with the following value: 28
tablets.
Note: The details of each individual manufactured item will be recorded in
the applicable manufactured item section (in this example those attributes
shall be recorded in other section includes the number of tablets per
manufacturing item and description including colour).
For liquid formulations requiring reconstitution (e.g., powder and
solvent for solution for injection), the unit of presentation shall be
differentiated, and this data field is repeated per manufactured item.
Example 3: powder (25 mg vial) and solvent (1 ml pre-filled syringe). In
this case the pack size field is repeated including quantity and unit of

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 134/231
Tag Description
presentation for the powder (vial) and quantity and unit of presentation
(syringe) for the solvent.
Value: 1 vial + 1 pre-filled syringe
The applicable numeric value(s) and unit of presentation shall be selected
from the term ID as listed in the applicable Referentials Management
Service (RMS) list.
Repeatable
Yes
Conformance
Mandatory
Data Type
Quantity
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000000014
Value(s) Numeric value and unit.
The units shall be specified as a Term ID listed in RMS Units of
Presentation list as applicable
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
containedItemQuantity
FHIR Path PackagedProductDefinition.extension.containedItemQuantity
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
Example(s):
20 tablets
1 vial (solvent) + 1 vial (powder)
1 tube (cream) + 27 tablets
150 (15 x 10 x 1) capsules (unit dose) (multipack)
Note: Content between brackets is for information and it is not included while completing this field.
4.4.1. Quantity operator (New)
Tag Description
User Guidance The applicable value corresponding to the quantity operator shall be
specified as term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000008
Value(s) As listed in the Quantity Operator RMS list. If the RMS term can be
mapped to a FHIR Quantity comparator, the FHIR comparator field should
also be specified for interoperability.
ISO Element Name
Not applicable

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 135/231
Tag Description
ISO Path
Not applicable
FHIR Element Name
Quantity Operator
FHIR Path PackagedProductDefinition.extension.containedItemQuantity.extension.qua
ntityOperator
PackagedProductDefinition.extension.containedItemQuantity.comparator
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.5. Legal status of supply
The legal status of the medicinal product's supply, as authorised by the competent authority in the
region and applicable to the individual package should be specified.
This section is only applicable where individual packages have different legal statuses of supply. In this
situation, legal status of supply at medicinal product level (section 1.7) should be populated with the
RMS term “Medicinal product subject to medical prescription exempt for some pack sizes”.
In cases where legal status of supply is identical for all package sizes of the medicinal product, this
field should be left empty, and the legal status of supply shall be reflected only at medicinal product
level (section 1.7).
Tag Description
User Guidance The legal status of the medicinal product's supply, as authorised by the
competent authority and applicable in the region, shall be specified using a
term ID.
The value(s) shall be selected from the term ID as listed in the applicable
Referentials Management Service (RMS) list.
• For CAPs, this information is retrieved from Annex II.B - CONDITIONS
OR RESTRICTIONS REGARDING SUPPLY AND USE and from section 4.2
Posology of the Product information
• For NAPs, this information may be retrieved from different sources
that includes from Product information (SmPC, Package Leaflet or
other annexes) to National Register of Medicinal Products.
• The legal status for the supply can be defined at packaged medicinal
product level. In this scenario it should be specified as Medicinal
product subject to medical prescription or Medicinal product not
subject to medical prescription.
Repeatable
No
Conformance
Conditional
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072051
Value(s)
As listed in the Legal Status for the Supply RMS list
ISO Element ID
LegalStatusOfSupply

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 136/231
Tag Description
ISO Path /MedicinalProduct/
PackagedMedicinalProduct/MarketingAuthorisation/LegalStatusOfSupply
FHIR Element ID
legalStatusOfSupply
FHIR Path
PackagedProductDefinition.legalStatusOfSupply
4.6. Marketing status
This section provides information on the marketing status of the packaged medicinal product. Marketing
status considers the concepts of placing in the market and market cessation. The terms “actual
marketing” and “placing on the market” should be defined as when the medicinal product is “released
into the distribution chain” e.g., out of control the Marketing authorisation holder. The “cessation of
placing on the market” shall be defined, by analogy to the placing on the market, as the “cessation of
release into the distribution chain” with the consequence that the concerned product may no longer be
available for the supply to the patients.
Therefore, the marketing status describes the date when a packaged medicinal product is available on
the distribution chain and on the market (placing in the market) or the date as of which it is no longer
available which is considered the date of the last release into the distribution chain (market cessation).
In the case of centralised authorised products (CAPs), this section needs to be completed for each
individual EU/EEA country.
This section considers the marketing status at the level of packaged medicinal product. As the concept
of marketing status for a medicinal product is linked to the moment that a medicinal product package is
released/removed from the market, it is important that information recorded at package medicinal
product level is accurate.
This section is to be completed when the information is available. Because the authorized medicinal
product can be placed on the market only after the product is authorized, this section should be populated
with the relevant information (when applicable) upon formal conclusion of the regulatory procedure of
marketing authorisation.
The data elements inside red rectangles apply for iteration 1 implementation:
Figure 26: Marketing Status (source: http://www.hl7.org/fhir
)
This class is mandatory and repeatable while the individual attributes of this class are not repeatable
and shall be populated as applicable.
Marketing status Class Description
Repeatable
Yes
Conformance
Mandatory

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 137/231
4.6.1. Country
Tag Description
User Guidance The country code of the country where the product is marketed/not
marketed should be specified as a term ID.
The value(s) shall be selected from the term ID as listed in the applicable
Referentials Management Service (RMS) list.
In the case of CAPs, all individual countries of the EU where the product is
marketed/not marketed should be selected.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000002
Value(s)
As listed in the Country RMS list
ISO Element Name
Country
ISO Path
/MedicinalProduct/PackagedMedicinalProduct/MarketingStatus/Country
FHIR Element Name
Country
FHIR Path
PackagedProductDefinition.marketingStatus.country
Example(s):
100000000373 – Republic of Croatia
100000000529 - Kingdom of Spain
4.6.2. Marketing status
Tag Description
User Guidance The status of the marketing of the medicinal product may be specified as a
term ID.
The value(s) shall be selected from the term ID as listed in the
Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072052
Value(s)
As listed in Marketing Status RMS list
ISO Element Name
Marketing Status
ISO Path
/MedicinalProduct/PackagedMedicinalProduct/MarketingStatus/Status
FHIR Element Name
Status
FHIR Path
PackagedProductDefinition.marketingStatus.status
Example(s):
Marketed, Not marketed, Temporarily unavailable

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 138/231
4.6.3. (Marketing status) start date
Tag Description
User Guidance The date when the authorised Medicinal Product is placed on the market in
a country, shall be provided by the Marketing Authorisation Holder (or
where applicable, the manufacturer/distributor).
Note “Placed on the market” is defined as the release of the authorised
Medicinal Product into the distribution chain i.e., out of direct control of the
Marketing Authorisation Holder.
This section is to be completed when the information is available.
Repeatable
No
Conformance
Conditional
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s)
A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
Marketing Date Start
ISO Path /MedicinalProduct/PackagedMedicinalProduct/MarketingStatus/MarketingDa
teStart,
FHIR Element Name
dateRange.start
FHIR Path
PackagedProductDefinition.marketingStatus.dateRange.start
4.6.4. (Marketing status) end date
Tag Description
User Guidance Note: “cessation of placing on the market” is defined, by analogy to the
placing on the market, as the “cessation of release into the distribution
chain” with the consequence that the concerned product may no longer be
available for the supply to the patients.
This section is only applicable when a medicinal product is removed from
the market. Otherwise, it is to be blank.
This section is to be completed when the information is available.
Repeatable
No
Conformance
Conditional
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s)
A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name Marketing Date Stop
ISO Path /MedicinalProduct/PackagedMedicinalProduct/MarketingStatus/MarketingDa
teStop

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 139/231
Tag Description
FHIR Element Name
dateRange.end
FHIR Path
PackagedProductDefinition.marketingStatus.dateRange.end
4.6.5. Risk of supply shortage
The information related to the risk of short and long-term shortages or other problems related to the
availability of medicines that can impact the medicine supply chain, can be reported through this data
element.
The risk of supply shortage refers to the potential risk of unavailability of the authorised medicinal
product marketed in a given member state of the European Union (EU).
The definition of “shortages” referred to in this guidance are to be understood in the context of the
harmonised definition agreed by EMA-HMA in the “Guidance on detection and notification of shortages
of medicinal products for Marketing Authorisation Holders (MAHs) in the Union (EEA)”:
‘A shortage of a medicinal product for human use occurs when supply does not meet demand at a
national level’.
The definition applies to all shortages that are already affecting or that are expected to affect one or
more EU member states in the future.
It applies to prescription and non-prescription medicines alike.
Tag Description
User Guidance
The indication on whether there is a risk of a product shortage shall be
specified. The risk of shortage is intended the potential risk to affect or is
likely to affect more than one European Union (EU) Member State where
the authorised medicinal product is marketed.
This data element should be specified when the marketing status is either
temporarily unavailable or not marketed.
Yes – The availability of the medicinal product can lead to the potential risk
of supply shortage in the EU member state in which the product is placed
on the market and released into the distribution chain.
No – The availability of the medicinal product does not lead to the potential
risk of supply shortage in the EU member state in which the product is
placed on the market and released into the distribution chain.
Repeatable
No
Conformance
Conditional
Data Type
Boolean
RMS URI/URL
Not applicable
Value(s) True / False
ISO Element Name
Risk of supply shortage

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 140/231
Tag Description
ISO Path /MedicinalProduct/PackagedMedicinalProduct/MarketingStatus/RiskOfSuppl
yShortage
FHIR Element Name
riskOfSupplyShortage
FHIR Path PackagedProductDefinition.marketingStatus.extension.riskOfSupplyShortag
e
4.6.6. Risk of supply shortage comment
Tag Description
User Guidance The risk of supply shortage comment attribute shall be used to indicate
any additional information related to the potential risk of supply shortage
of authorised medicinal products within a given member state of the
European Union, where the product is placed on the market and released
into the distribution chain.
This data element should be specified when the marketing status is either
temporarily unavailable or not marketed.
Repeatable
No
Conformance
Conditional
Data Type
Markdown
RMS URI/URL
Not applicable
Value(s)
The details about the risk of supply shortage shall be provided as text.
ISO Element Name
Risk of supply shortage comment
ISO Path /MedicinalProduct/PackagedMedicinalProduct/MarketingStatus/RiskOfSuppl
yShortageComment
FHIR Element Name
riskOfSupplyShortageComment
FHIR Path PackagedProductDefinition.marketingStatus.extension.riskOfSupplyShortag
eComment
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.6.7. Status reason
The Status Reason class describe the reason of the legal action taken on the marketing with reference
to the attribute 4.5.2 Marketing status in case of any change to the availability of the authorised
medicinal product on the market of the given European Union (EU) member state.
This applies to cases when the product is temporarily unavailable (i.e., temporarily cessation) or
withdrawal of the product from the market (i.e., non marketed).
The information specified in this class can support the management of risk of supply shortage across
the European Union.
This class and its individual attributes are conditional and not repeatable.
Status Reason Class Description
Repeatable
No

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 141/231
Status Reason Class Description
Conformance
Conditional
4.6.7.1. Reason
Tag Description
User Guidance The information related to the reason of any action taken on the
unavailability of the authorised medicinal product on the market of the
given European Union (EU) member state shall be specified to prevent the
risk of supply shortage.
Information shall be provided in cases when the authorised medicinal
product is temporarily unavailable (i.e., temporarily cessation) or
withdrawal of the product from the market (i.e., non marketed).
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000018799
Value(s)
As listed in the Reason for Marketing Unavailability RMS list
ISO Element Name
Reason
ISO Path /MedicinalProduct/PackagedMedicinalProduct/MarketingStatus/StatusReaso
ns/Reason
FHIR Element Name
reason
FHIR Path PackagedProductDefinition.marketingStatus.extension.reason
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
Example(s):
• Safety - Medicine is harmful (Art. 116, 117(1a) of Directive No 2001/83/EC)
• Efficacy – Lack of efficacy (Art. 116, 117(1b) of Directive No 2001/83/EC)
• Risk/benefit - Not favourable (Art. 116, 117(1c) of Directive No 2001/83/EC)
• [..]
4.6.7.2. Restore date
Tag Description
User Guidance The date when the authorised medicinal product is estimated to be
reintroduced into the market of the given European Union (EU) member
state can be provided.
The estimated date or reintroduction applies only for temporary cessations
of the authorised medicinal product.
Repeatable
No
Conformance
Optional
Data Type
dateTime
RMS URI/URL
Not applicable
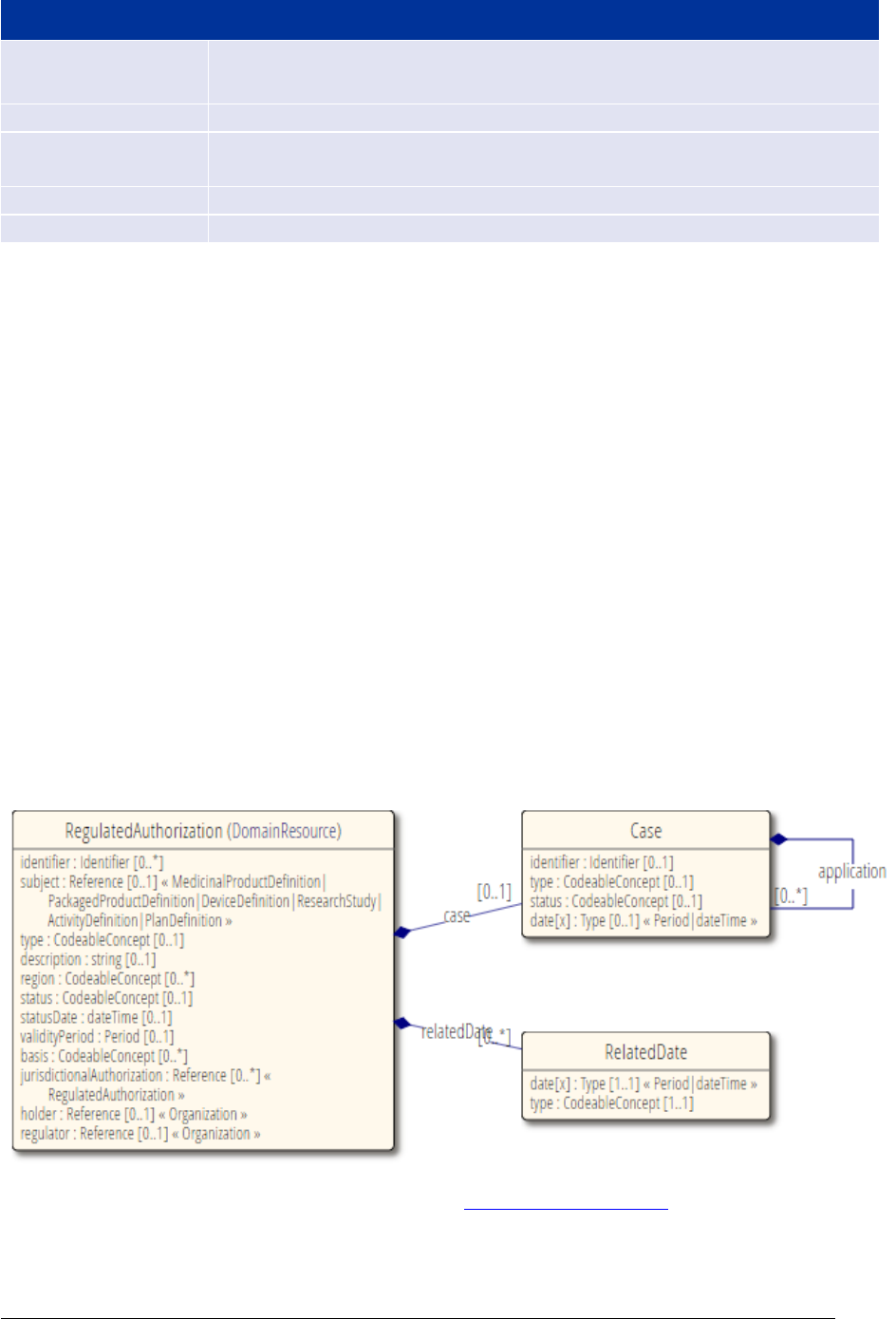
Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 142/231
Tag Description
Value(s)
A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
RestoreDate
ISO Path /MedicinalProduct/PackagedMedicinalProduct/MarketingStatus/StatusReaso
ns/RestoreDate
FHIR Element Name
restoreDate
FHIR Path
PackagedProductDefinition.marketingStatus.restoreDate
4.7. Marketing authorisation (Package level)
There are cases where marketing authorisation is assigned at the level of packaged medicinal product.
If any information related to the Marketing Authorisation be regulated by the applicable National
Competent Authority at the level of the individual pack of the medicinal product and be different for
the other packages (i.e., different from the entire medicinal product), the applicable information shall
be specified according to the FHIR Resource RegulatedAuthorization and guidance provided in section
2. Marketing authorisation information.
If the marketing authorisation number or equivalent identifier is assigned at package level, the ‘root’
number (e.g., EU/1/YY/NNNN) only is provided at the level of medicinal product (as described in
section 2.2. ). The individual package authorisation number part shall be specified for the individual
package in this section including the root number.
If the authorisation number or equivalent identifier is assigned at Packaged Medicinal Product level and
no stable “root number” common to all packaged medicinal products is assigned, the marketing
authorisation number is to be left blank at medicinal product level (as described in section 2.2. ).
Also, it is mandatory to provide the marketing authorisation number at packaged medicinal product
level as described in this section.
Figure 27: Resource RegulatedAuthorization (source: http://www.hl7.org/fhir)
The Marketing authorisation (Package level) class is a mandatory and not repeatable while the
individual attributes of this class are not repeatable and shall be populated as applicable.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 143/231
Marketing authorisation (Package level)
Class
Description
Repeatable
No
Conformance
Mandatory
4.7.1. Regulatory authorisation type
Tag Description
User Guidance
The type of regulatory authorisation shall be specified when the marketing
authorisation is assigned at the level of packaged medicinal product.
Please note that the RegulatedAuthorization type shall be set as Marketing
Authorisation
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Conditional
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/220000000060
Value(s) • As listed in the Regulatory Entitlement Type RMS list
• The RMS term “Marketing Authorisation” shall be selected.
ISO Element Name
Not Applicable
ISO Path
Not Applicable
FHIR Element Name
Type
FHIR Path
RegulatedAuthorization.type
4.7.2. Marketing authorisation number (Package level)
Tag Description
User Guidance
Marketing Authorisation number or equivalent identifier as assigned by the
component authority to the medicinal product package shall be specified.
This section is to be completed when the information is available Because
the Marketing Authorisation Number or equivalent identifier is released by
the competent authority after the end of the regulatory procedure, this
data field can only be populated with the necessary information upon
formal conclusion of the regulatory procedure as this information will not
be available at the time of the submission of the FHIR message (initial
sequence).
Repeatable
No
Conformance
Conditional
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s) The number assigned by the competent authority of a country/jurisdiction
shall be specified as free text.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 144/231
Tag Description
• The format of the EU number shall be "EU/1/YY/NNN/XXX " or
"EU/1/YY/NNNN/XXX" (as applicable)
ISO Element Name
Marketing Authorisation Number
ISO Path /MedicinalProduct/PackagedMedicinalProduct/MarketingAuthorisation/Mark
etingAuthorisationNumber
FHIR Element Name
Identifier
FHIR Path
RegulatedAuthorization.identifier
FHIR Complementary
Information
RegulatedAuthorization.identifier.system value is
http://ema.europa.eu/fhir/marketingAuthorizationNumber
4.7.3. Country
Tag Description
User Guidance The country code of the country where the marketing authorisation of the
packaged medicinal product was granted, shall be specified as a term ID.
• The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
• For medicinal products authorised via the centralised procedure,
"European Union (EU)" shall be specified.
• For medicinal products authorised in Liechtenstein, Norway and Iceland
via the centralised procedure, the applicable country code (i.e.,
LI/NO/IS) shall be specified.
• For medicinal products authorised via national or MRP/DCP procedure,
the applicable EEA country shall be specified.
• For medicinal products authorised outside the EU/EEA area, a non-EEA
country shall be specified.
• Medicinal products authorised outside the EU/EEA area may be
submitted on voluntary basis. Such medicinal products are not within
the scope of Article 57(2) requirements.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000002
Value(s)
As listed in the Country RMS list
ISO Element Name
Country
ISO Path /MedicinalProduct/PackagedMedicinalProduct/MarketingAuthorisation/Coun
try
FHIR Element Name
Region
FHIR Path
RegulatedAuthorization.region
Example(s):
100000000529 – Kingdom of Spain

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 145/231
100000000390 - European Union
4.7.4. Authorisation status
Tag Description
User Guidance
The status of the marketing authorisation of the packaged medicinal
product throughout its lifecycle shall be specified as a term ID.
• The applicable value shall be selected from the term ID as listed in
applicable the Referentials Management Service (RMS) list.
• The value “Valid after lifting of suspension” shall be entered when the
marketing authorisation returned to status valid after the suspension
of a marketing authorisation has been lifted.
• The value “Valid - Renewed” shall be entered when the marketing
authorisation is renewed.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072049
Value(s)
As listed in the Regulatory Entitlement Status RMS list
ISO Element Name
Authorisation Status
ISO Path /MedicinalProduct/PackagedMedicinalProduct/MarketingAuthorisation/
AuthorisationStatus
FHIR Element Name
Status
FHIR Path
RegulatedAuthorization.status
Example(s):
Withdrawn
Not Renewed
Expired
Revoked
Valid
4.7.5. Authorisation status date (Package level)
Tag Description
User Guidance The date at which the authorisation status of the package medicinal
product has become effective shall be specified.
This data element is to be completed when the information is available.
• The date when the first authorisation was granted by the authorising
body or the date when the renewal was granted (whichever is the
latest) shall be specified in line with section 9. Date of first

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 146/231
Tag Description
authorisation/renewal of the authorisation of the SmPC or any other
regulatory document.
• The date of expiry of the MA shall be specified if the MA was:
− withdrawn by the MAH;
− revoked by the competent authority;
− not renewed by the competent authority;
− not submitted for renewal by the MAH;
− expired due to Sunset Clause.
• The date of suspension of the MA shall be entered if the authorisation
status is set to Suspended.
• The date of renewal of the MA shall be entered if the authorisation
status is set to Valid – after renewal
• In case that status “Valid after lifting of suspension is selected” in
previous attribute the date of lifting of the suspension shall be entered.
Repeatable
No
Conformance
Conditional
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s) A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
Authorisation Status Date
ISO Path /MedicinalProduct/PackagedMedicinalProduct/MarketingAuthorisation/
AuthorisationStatusDate
FHIR Element Name
statusDate
FHIR Path
RegulatedAuthorization.statusDate
4.8. Package item (container)
The description of the container(s) of the manufactured medicinal product shall be described in this
section. Container can be described as an item holding or carrying another item of the medicinal
product. Therefore, containers can involve the primary or secondary packaging of the medicinal
product.
The package item can be a single item, or a package of multiple items (e.g., two vials contained in a
carton box, three blisters contained in a carton box or a single individual bottle).
For instance, a Packaged medicinal product consisting of a blister within a box will contain two Package
items. One Package item will be the carton box, and the second container will be the blister, which is
considered a sub-container.
Another example corresponds with a carton box (secondary packaging) containing two vials; solvent
and powder (primary packaging). This packaged medicinal product contains therefore three package
item containers (1) carton, (2) vial containing powder and (3) vial containing solvent.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 147/231
Reference on this recursive relationship needs to be included accordingly as reflected in Manufacturing
item reference(s).
A more detailed example is included below.
Example(s):
Packaged Medicinal Product
PCID EU-00001457-00040001-0001
Package Description Pack of one vial (type I glass) of 2.5 ml suspension with a
stopper (butyl rubber) and one vial (type I glass) of 2.5ml
emulsion with a stopper (butyl rubber)
Package Item Container (1) Box
Package Item Container Quantity 1
Package Item Material Recycled Cardboard
Packaged Item Reference Reference to Package item 2 and Package item 3
Manufacturing item Reference Not Applicable
Data Carrier Identifier (s) 02890138016090
Package Item Container (2) Vial
Package Item Container Quantity 1
Package Item Material Glass type I
Package Item Component Type Vial
Package Item Component
Material
Glass type I
Package Item Component Type Stopper
Package Item Component
Material
Butyl Rubber
Manufactured Item
(Manufactured Dose Form)
Suspension for emulsion for injection
Packaged Item Reference Not applicable
Manufacturing item Reference Reference to Suspension for Emulsion for Injection
Package Item Container (3) Vial
Package Item Container Quantity 1
Package Item Material Glass type I

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 148/231
Package Item Component Type Vial
Package Item Component
Material
Glass type I
Package Item Component Type Stopper
Package Item Component
Material
Butyl Rubber
Manufactured Item
(Manufactured Dose Form)
Emulsion for emulsion for injection
Packaged Item Reference Not applicable
Manufacturing item Reference Reference to Emulsion for Emulsion for Injection
The Package item (container) class is a mandatory and repeatable. Some of the individual attributes of
this class are not repeatable and shall be populated as applicable with the exception of package item
(container) type and material data elements which are mandatory.
Package item (container) Class Description
Repeatable
Yes
Conformance
Mandatory
4.8.1. Package item (container) type
Tag Description
User Guidance This element describes the physical type of the container of the medicinal
product and shall be specified using a term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000073346
Value(s)
As listed in the Packaging RMS list
ISO Element name
Package item (container) type
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Pack
ageItem_ContainerType
FHIR Element name
Type
FHIR Path
PackagedProductDefinition.package.type
Example(s):
Bottle
Blister

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 149/231
Box
Vial
Blister Sleeve
Pre-filled Syringe
4.8.2. Package item reference(s)
Relationships among package items in the scenario that a package item container (e.g., carton,
secondary packaging) contains one or more package item such as additional secondary packaging or
primary packaging only (e.g., vials or blisters within a carton) needs to be indicated.
This structure is only applicable in a parent/child relationship and not the opposite (child/parent
relationship). This means that the relationship is established at the level of the package item acting as
a container for other package container and not the opposite.
Tag Description
User Guidance This is not a regular attribute but a reference to a list of package items
contained within a package item (if applicable).
For example, a cardboard carton box that contains 2 vials will carry the
reference to the 2 vials contained within the box.
This is also applicable to reference the relationships of a single or multiple
component contained within a package container.
For example, a container vial that contains a component stopper (butyl
rubber) vial will carry the reference to the butyl rubber stopper.
This field is only applicable in a parent/child relationship and not the
opposite (child/parent relationship).
Repeatable
Yes
Conformance
Conditional
Data Type
BackboneElement
RMS URI/URL
Not applicable
Value(s)
Structured data elements describing the included packages.
ISO Element name
Package item (container)
ISO Path
/MedicinalProduct/PackagedMedicinalProduct/PackageItemContainer/
FHIR Element name
Package
FHIR Path
PackagedProductDefinition.package.package
Example(s):
Carton containing two vials
This is a simplified JSON version
"packageItem" : [{
"identifier" : 123
"type" : { Cardboard Box },
"quantity" : { 1 },
"packageItem" : [{

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 150/231
"identifier" : 456
"type" : { Vial },
"quantity" : { 2 }, }]
}]
4.8.3. Manufactured item reference(s)
Relationships among package items and manufactured items need to be completed. This attribute is
for mandatory completion where the package item is/contains the manufacturing item (e.g., a vial
containing the pharmaceutical form powder for solution for injection).
If the package item does not contain any manufactured item (e.g., empty vial, carton), this field is to
be left blank.
Tag Description
User Guidance This is not a regular attribute but a reference to a manufactured item
contained within a package item (if applicable)
For example, a vial containing powder will carry the reference to
manufacturing item powder for solution for injection.
This structure is only applicable at the level of package item involves the
primary packaging and contains a manufacturing item
Repeatable
Yes
Conformance
Conditional
Data Type
BackboneElement
RMS URI/URL
Not applicable
Value(s) Structured data elements referencing the included manufactured items and
the respective quantities.
ISO Element name
Manufactured item
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItemContainer/Manuf
acturedItem
FHIR Element name
Containeditem
FHIR Path
PackagedProductDefinition.package.containeditem
4.8.4. Device reference(s)
A packaged medicinal product may include medical devices for different purposes (e.g., administration
device).
Relationships among package items and medical devices need to be completed where applicable. This
attribute is for mandatory completion where the package medicinal product contains a medical device.
Two situations can be foreseen:
• The medical device is included as independent elements within the packaged medicinal product
(e.g., spoon, syringes, pens) – In this case the relationship of the medical device with the package
item is established at least one-level above the primary packaging (e.g., carton).
• The medical device is integrated and contains the medicinal product already as packaged for sale
(e.g., pre-filled syringes, pre-filled pen) - In this case the relationship of the medical device with
the package item is established at the level of the primary packaging (e.g., pen, syringe). As

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 151/231
consequence, the device (e.g., pen, syringe) will be both a package item container and a medical
device.
If there is no medical device included within the packaged medicinal product, this field is to be left
blank.
Tag Description
User Guidance This is not a regular attribute but a reference to two possible situations
• Medical device contained within a package item (e.g., a spoon
contained within the secondary packaging of the medicinal product)
• A medical device which is also the primary packaging/package item
container for the medicinal product (e.g., pre-filled syringe)
This structure is only applicable if there is a medical device included in the
packaged medicinal product.
Repeatable
Yes
Conformance
Conditional
Data Type
BackboneElement
RMS URI/URL
Not applicable
Value(s) Structured data elements referencing the included devices and the
respective quantities.
ISO Element name
Device
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItemContainer/Devic
e
FHIR Element name
ContainedItem
FHIR Path PackagedProductDefinition.package.containedItem
4.8.5. Package item (container) quantity
The number of the package item containers shall be specified. Because the package item class may
contain recursive relationships to describe containers within containers, the first container (top-level
package item/outer-most packaging) will always have a quantity of one (i.e., '1').
Example 1: Medicinal product A 500mg tablets with 30 tables in 3 blisters (10 tablets per blister)
packaged in a single carton box.
• Carton x 1
o Blister x 3
There is no need to indicate the number of tablets since this information is included in section 4.11.2.
Manufactured item quantity.
Note: If the same packaged medicinal product contains different configurations (e.g., Medicinal Product
ABC 500mg 30 tablets contained in either 2 blisters of 15 tablets each or 3 blisters of 10 tablets per
blister) which are not defined in the terms of the marketing authorisation or packaged description,
Package item (container) quantity at the level of the immediate packaging is to be left blank.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 152/231
Example 2: Medicinal product B comprises combined pharmaceutical form powder (40 micrograms)
and solvent (1 ml) for solution for injection. This package example consists of two vials with solvent
and two vials with powder, all vials packaged in a single carton box.
• Carton x 1
o Vial (solvent) x 2
o Vial (powder) x 2
There is no need to indicate the content of each vial since this information is included in section
Manufactured Item.
Tag Description
User Guidance
The number of the package item shall be specified, when applicable.
The first container (top-level package Item/outer packaging) will always
have a quantity of one (i.e., '1').
Repeatable
No
Conformance
Conditional
Data Type
Quantity
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000000014
Value(s) Quantity value and unit.
The units shall be specified as a Term ID as listed in the Units of
Presentation RMS list.
ISO Element Name
Package item (container) quantity
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Pack
ageItem_ContainerQuantity
FHIR Element Name
Quantity
FHIR Path
PackagedProductDefinition.package.quantity
4.8.5.1. Quantity operator (New)
Tag Description
User Guidance The applicable value corresponding to the quantity operator shall be
specified as term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000008
Value(s) As listed in the Quantity Operator RMS list. If the RMS term can be
mapped to a FHIR Quantiy comparator, the FHIR comparator field should
also be specified for interoperability.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Quantity Operator
FHIR Path PackagedProductDefinition.package.quantity.extension.quantityOperator
PackagedProductDefinition.package.quantity.comparator

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 153/231
Tag Description
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.8.6. Data carrier identifier
When using different packaging levels of medicinal products (e.g., secondary packaging, primary
packaging), data carrier identifiers (e.g., barcodes) and the type of identifier may be specified. For the
purpose of this iteration, only the data carrier identifier in the outer-most package should be specified.
In the European context, should the medicinal product fall within the scope of the EU Falsified
Medicines Directive - FMD (Directive 2011/62/EU
) which amended Directive 2001/83/EC, the Identifier
available in the central information may be specified. This is the value uploaded by the MAH to the
EMVS database (‘hub’) as referred to in Article 32 of the
Commission Delegated Regulation (EU)
2016/161.
The data carrier identification number of the outer-most packaging of the packaged medicinal product
shall be specified using the Global Trade Item Number (GTIN) or National Trade Item Number (NTIN)
identification system as recorded in the European Medicines Verification System (EMVS) or the
Pharmacy Product Number (PPN). Rules regarding GTIN or NTIN are specified in EMVS annex 2.
In case of multiple identifiers per PCID (e.g., language versions), all the relevant identifiers shall be
specified.
The set of Data carrier identifier attributes are optional and repeatable.
Attachment document Class Description
Repeatable Yes
Conformance Optional
4.8.6.1. Identifier value (New)
Tag Description
User Guidance The outer-most packaging carrier identifier (GTIN/NTIN/PPN) may be
specified.
e.g.,
Repeatable
No
Conformance
Mandatory
Data Type
String
RMS URI/URL
Not applicable
Value(s)
String
ISO Element Name
Data Carrier Identifier
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Data
CarrierIdentifier
FHIR Element Name

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 154/231
Tag Description
value
FHIR Path
PackagedProductDefinition.package.identifier.value
Example(s):
GTIN: 028901563801609
PPN: 30 A123456789 09
NTIN: 0 90 8985 391109 8
Note: Examples are fictitious
4.8.6.2. Identifier system (New)
Tag Description
User Guidance The source system relevant to the reported identifier can be specified.
The applicable term shall be provided as a term ID from the Source of
Information RMS list.
Repeatable
No
Conformance
Mandatory
Data Type
URI
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000009
Value(s) URI referencing to the applicable RMS term from the Source of Information
RMS list (i.e., https://spor.ema.europa.eu/v1/lists/100000000009
/terms/100000075665).
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
system
FHIR Path
PackagedProductDefinition.package.identifier.system
Example(s):
GS1 Global Trade Item Number (100000167575)Pharmacy Product Number (200000027029)
National Trade Item Number (200000027030)
4.8.7. Material
Tag Description
User Guidance The material the package item is made from shall be specified using a
term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
If several materials are identified in the SmPC (e.g., Aluminium, PVC for
blisters) this field needs to be repeated listing all relevant materials.
Repeatable
Yes
Conformance
Mandatory

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 155/231
Tag Description
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000003199
Value(s)
As listed in the Material RMS list
ISO Element name
Material
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Mate
rial
FHIR Element name
Material
FHIR Path
PackagedProductDefinition.package.material
Example(s):
Paper, plastic, glass
4.9. Package (component)
This class shall be used to further describe any part of the packaging of the packaged medicinal
product. The package item container (primary packaging) may be described with additional
components of the container (e.g., closure and seal of vials, needle guards). In particular components
with an impact on the drug product's stability shall be reported (example: desiccant). This applies
occur either when the desiccant is part of the package item (i.e., desiccant included in the bottle) or
when the desiccant is include as separate item (i.e., desiccant bag).
The description can be of a complete container or a part of a container, such as a closure.
If the indicated package container corresponds to the primary packaging (direct contact with the
product itself) the same term used to describe the packaged component type shall be applied to
indicate the packaged component type.
If the indicated packaged container does not correspond to the primary packaging the relevant term
shall not be repeated as packaged (component) type in PMS. Refer to Chapter 8 Annex I – Complete
representation.
This information should be provided as part of the submission.
The information in section 6.5 of the SmPC should be used as a basis for the description of the
package components. Two examples are included below to illustrate this concept.
Example(s):
Description Cartridge (type 1 glass) with a plunger
(bromobutyl) and a laminate rubber sheet
(bromobutyl/polyisoprene) contained in a pre-
filled multidose disposable pen made of
polyolefin and polyacetal.
Package Item Container Cartridge in a pre-filled pen
Package Item Container Material Glass type I
Package Item Component Type (1) Plunger

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 156/231
Package Item Component Material (1) Bromobutyl
Package Item Component Type (2) Rubber sheet
Package Item Component Material (2) Bromobutyl/polyisoprene
Note: Polyolefin and polyacetal are materials of the device and not the package item container and
should not be captured.
Example(s):
Description 30 ml of concentrate in a vial (Type I glass) with
a stopper (butyl, siliconised), and a seal
(aluminium) with flip-off cap (polypropylene).
Package Item Container Vial
Package Item Container Material Glass type I
Package Item Component Type (1) Vial
Package Item Component Material (1) Glass type I
Package Item Component Type (2) Stopper
Package Item Component Material (2) butyl, siliconized
Package Item Component Type (3) Seal
Package Item Component Material (3) Aluminium
Package Item Component Type (4) Cap
Package Item Component Material (4) Polypropylene
The Package (component) class is mandatory and repeatable while the individual attributes of this
class are not repeatable (with the exception of component material data element) and shall be
populated as applicable.
Package item (component) Class Description
Repeatable
Yes
Conformance
Mandatory
4.9.1. Component type
Tag Description
User Guidance
The type of component, whose material is being described, shall be
specified.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 157/231
Tag Description
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000073346
Value(s)
As listed in the Packaging RMS list
ISO Element name
Component Type
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Pack
age_Component/ComponentType
FHIR Element name
Type
FHIR Path
PackagedProductDefinition.package.package.type
Example(s):
Child-Resistant Closure
Stopper
Plunger
4.9.2. Component material
Tag Description
User Guidance The material(s) of the component may be specified using a term ID, as
applicable.
The applicable value(s) shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
If several different materials are identified for the same component, this
field needs to be repeated listing all relevant materials.
Repeatable
Yes
Conformance
Conditional
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000003199
Value(s)
As listed in the Material RMS list
ISO Element name
Component Material
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Pack
age_Component/ComponentMaterial
FHIR Element name
Material
FHIR Path
PackagedProductDefinition.package.package.material
Example(s):
Paper, plastic, glass
4.10. Medical device
As defined in the Directive 2001/83/EC, medicinal products can be marketed for use in combination
with a medical device, usually to enable the delivery of the medicine. Information on the medical

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 158/231
device, based on the elements described in this section should be provided when the medicinal product
is presented in combination with medical devices. Overall, there are two types of combination:
• integral: the medicinal product and device form a single integrated product e.g., pre-filled syringes
and pre-filled pens, certain types of patches for transdermal drug delivery and pre-filled inhalers;
• co-packaged: the medicinal product and the device are separate items contained in the same pack
e.g., reusable pen for insulin cartridges, tablet delivery system with controller for pain
management.
In addition, the device may be combined with the medicinal product and support the
pharmacological/metabolic/immunological action of the medicinal product (collagen scaffold). In these
cases, the device’s purpose goes beyond the administration and supports the mechanism of action of
the medicinal product. These types of devices are considered an integral part of the pharmaceutical
product administered to the patient and should be recorded in this section as well as section 6.5
Medical Device.
The full information on Device as presented in the FHIR Resource DeviceDefinition is shown in the
figure 18
below. In Iteration 1 of the PMS implementation, the elements inside the red rectangles are
in scope and shall be provided according to the rules and guidance described in this section:
Figure 28: Resource DeviceDefinition (Source : http://www.hl7.org/fhir/
)
The Device class is conditional and repeatable while the individual attributes of this class are not
repeatable and shall be populated as applicable.
Device Class Description
Repeatable
Yes
Conformance
Conditional

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 159/231
4.10.1. Type of medical device used in combination with medicinal product
Tag Description
User Guidance The type of medical device used in combination with medicinal product
shall be specified when using a term ID if applicable.
The applicable value shall be selected from the term ID listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/rmswi/#/lists/200000025965/
Value(s)
As listed in the Medical Device Legislative Category RMS list
ISO Element name
DeviceType
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Devi
ce
FHIR Element name
typeOfCombination
FHIR Path
DeviceDefinition.extension.typeOfCombination
Example(s):
Integral – Not administration device (200000025966)
Integral – Administration device (200000025967)
Combined advanced therapy medicinal product (200000025968)
Co-packaged (200000025969)
Referenced in the product information of the medicinal product (200000025970)
Companion diagnostic (200000025971)
4.10.2. Medical device type
Tag Description
User Guidance The Type of the device or device system of the medicinal product shall be
specified when using a term ID if applicable.
The applicable value shall be selected from the term ID listed in in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance Based on the following terms specified in 4.10.1.:
• Mandatory:
- Integral – Not administration device
- Integral – Administration device
- Combined Advanced Medicinal Product Therapies (ATMP)
- Co-packaged
- Referenced in the PI of the medicinal product

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 160/231
Tag Description
- Companion diagnostic (IVD)
Data Type
CodeableConcept
RMS URI/URL
The applicable value as listed from the RMS list [list will be created in
v2.2]
Value(s)
As listed in the Medical Device Type RMS list
ISO Element name
Device Type
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Devi
ce/DeviceType
FHIR Element name
Type
FHIR Path
PackagedProductDefinition.manufacturer type
Example(s):
Measuring spoon, cup, cannula, spatula, injection needle, injection syringe,
4.10.3. Medical device identification
Article 27 and Article 28 of the New Medical Device Legislation (EU) 2017/745 establishes an electronic
system for the unique device identification. This facilitates the identification and traceability of devices
and the establishment of a European Database on Medical Devices (EUDAMED). This database is
currently under development by the European Commission.
The Unique Device Identifier (UDI), is a series of numeric or alphanumeric characters that is created
through a globally accepted device identification and coding standard. It allows the unambiguous
identification of a specific device on the market.
Integrated devices may be governed by the Medicinal Products Directive (EC) 2001/83, and therefore
the MDR obligations related to UDI may not be required and are not be applied to the package of the
combination product. The UDI may be assigned to integral devices with CE mark. Overall, the UDI shall
be provided where available and applicable.
The Unique Device Identifier (UDI) shall be specified when available.
Tag Description
User Guidance
The Unique Device Identifier (to the level of UDI device identifier (UDI-DI)
at the level of the medical device model recorded in the European
database on medical devices (EUDAMED) shall be specified, if applicable,
to the medicinal product.
Note: The Unique Device Identifier at the level of batch/product (UDI-PI)
shall not be provided
Repeatable
No
Conformance Based on the following terms specified in 4.10.1.:
• Conditional:
− Integral – Not administration device
− Integral – Administration device
− Combined Advanced Medicinal Product Therapies (ATMP)
− Co-packaged

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 161/231
Tag Description
• Not applicable:
− Referenced in the PI of the medicinal product
− Companion diagnostic (IVD)
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
Unique Device Identifier
ISO Element name
Device Identifier
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Devi
ce/DeviceIdentifier
FHIR Element name
Identifier
FHIR Path
DeviceDefinition.identifier
FHIR Complementary
Information
DeviceDefinition.identifier.system value to be defined based on RMS term
entry for Source of Information RMS list.
4.10.4. Medical device trade name
Tag Description
User Guidance
The name of the device should be specified, if applicable, as recorded in
the European database on medical devices (EUDAMED) or as recorded in
medicinal product regulatory submission and SmPC for medical devices
falling under Directive (EC) 2001/83.
Repeatable
No
Conformance Based on the following terms specified in 4.10.1.:
• Conditional:
− Integral – Not administration device
− Integral – Administration device
− Combined Advanced Medicinal Product Therapies (ATMP)
− Co-packaged
− Referenced in the PI of the medicinal product
−
Companion diagnostic (IVD)
Data Type
String
RMS URI/URL
Not applicable
Value(s)
Medical Device Trade Name
ISO Element name
Device Trade Name
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Devi
ce/DeviceTradeName
FHIR Element name
Name
FHIR Path
DeviceDefinition.deviceName.name
FHIR Complementary
Information
DeviceDefinition.deviceName.type value is “other”
Example(s):
OptiKlack

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 162/231
InsuAll
Note: Examples are fictitious
4.10.5. Medical device quantity
Tag Description
User Guidance The quantity (number of units) of the device(s) in the medicinal product
package, shall be specified as a value and units (as per section 6.5 of the
SmPC).
If RMS list “Unit of presentation” does not contain the applicable type of
device, the term “countable unit(s)” from Unit of Measurement List shall be
selected.
Repeatable
No
Conformance Based on the following terms specified in 4.10.1.:
• Mandatory:
− Integral – Not administration device
− Integral – Administration device
− Combined Advanced Medicinal Product Therapies (ATMP)
− Co-packaged
• Not applicable:
− Referenced in the PI of the medicinal product
− Companion diagnostic (IVD)
Data Type
Quantity
RMS URI/URL • https://spor.ema.europa.eu/v1/lists/100000110633
•
https://spor.ema.europa.eu/v1/lists/200000000014
Value(s) Numeric value and unit.
The units shall be specified as a Term ID listed in the Units of
Measurement List or Units of Presentation list as applicable
ISO Element name
DeviceQuantity
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Devi
ce/DeviceQuantity
FHIR Element name
amount
FHIR Path
PackagedProductDefinition.package.containedItem.amountQuantity
4.10.5.1. Quantity operator (New)
Tag Description
User Guidance The applicable value corresponding to the quantity operator shall be
specified as term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 163/231
Tag Description
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000008
Value(s) As listed in the Quantity Operator RMS list. If the RMS term can be
mapped to a FHIR Quantity comparator, the FHIR comparator field should
also be specified for interoperability.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
quantityOperator
FHIR Path PackagedProductDefinition.package.containedItem.amountQuantity.extensi
on.quantityOperator
PackagedProductDefinition.package.containedItem.amountQuantity.compa
rator
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.10.6. Medical device description (New)
Tag Description
User Guidance
The high-level description of the applicable medical device shall be
reported in this data element.
Information comprehensive of the different and single component(s) of the
medical device used (i.e., smart tools etc) shall also be provided, when
available.
Products authorised through MRP/DCP/NP routes
• The medical device description is to be provided in English or in the
local language(s) of authorisation, or optionally in all of them.
Products authorised through the centralised procedure
The medical device description is to be provided in English.
Repeatable
Yes
Conformance Based on the following terms specified in 4.10.1.:
• Mandatory:
− Integral – Not administration device
− Integral – Administration device
− Combined Advanced Medicinal Product Therapies (ATMP)
− Co-packaged
− Referenced in the PI of the medicinal product
− Companion diagnostic (IVD)
Data Type
Markdown
RMS URI/URL
Not applicable

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 164/231
Tag Description
Value(s)
Free text or markdown text for rich content
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
description
FHIR Path DeviceDefinition.extension.description
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.10.6.1. Language (New)
This section described how to populate information related to the language of the medical device
description. The provision of the language is mandatory.
Tag Description
User Guidance The language of the medical device description as specified in previous
section shall be specified.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072057
Value(s)
As listed in the Language RMS list
ISO Element Name
Not Applicable
ISO Path
Not Applicable
FHIR Element Name
valueCode
FHIR Path DeviceDefinition.extension.description.extension.language
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.10.7. Medical device description of intended purpose (New)
Tag Description
User Guidance The description of the intended purpose of the type of medical device shall
be reported in this data element.
The description of the intended purpose shall reflect the text as reported in
the CE certificate released by the relevant Notified Body.
Products authorised through MRP/DCP/NP routes
• The medical device description of intended purpose is to be provided in
English or in the local language(s) of authorisation, or optionally in all
of them.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 165/231
Tag Description
Products authorised through the centralised procedure
The medical device description of intended purpose is to be provided in
English.
Repeatable
Yes
Conformance Based on the following terms specified in 4.10.1.:
• Mandatory:
− Integral – Not administration device
− Integral – Administration device
− Combined Advanced Medicinal Product Therapies (ATMP)
− Co-packaged
− Referenced in the PI of the medicinal product
− Companion diagnostic (IVD)
Data Type
Markdown
RMS URI/URL
Not applicable
Value(s)
Free text or markdown text for rich content
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
intendedPurpose
FHIR Path DeviceDefinition.extension.intendedPurpose
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.10.7.1. Language (New)
This section described how to populate information related to the language of the medical device
description. The provision of the language is mandatory.
Tag Description
User Guidance The language of the medical device description as specified in previous
section shall be specified.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072057
Value(s)
As listed in the Language RMS list
ISO Element Name
Not Applicable
ISO Path
Not Applicable
FHIR Element Name
valueCode
FHIR Path
DeviceDefinition.extension.intendedPurpose.extension.language

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 166/231
Tag Description
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.10.8. Medical device classification (New)
Tag Description
User Guidance The relevant classification of the type of medical device shall be specified
by using a term ID, as applicable.
The applicable value(s) shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance Based on the following terms specified in 4.10.1.:
• Mandatory:
− Integral – Not administration device
− Integral – Administration device
− Combined Advanced Medicinal Product Therapies (ATMP)
− Co-packaged
− Referenced in the PI of the medicinal product
• Not applicable:
−
Companion diagnostic (IVD)
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/rmswi/#/lists/200000025960
Value(s)
As listed in the Medical Device Classification RMS list
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
classification
FHIR Path DeviceDefinition.extension.classification
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
Example(s):
Class I
Class IIa
Class IIb
Class III

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 167/231
4.10.9. Medical device manufacturer (New)
Tag Description
User Guidance
The reference to the relevant Manufacturer of the medical device shall be
selected from the list of manufacturers recorded in section 1.20
Manufacturing business operation.
Repeatable
Yes
Conformance Based on the following terms specified in 4.10.1.:
• Mandatory:
− Integral – Not administration device
− Integral – Administration device
− Combined Advanced Medicinal Product Therapies (ATMP)
− Co-packaged
− Referenced in the PI of the medicinal product
− Companion diagnostic (IVD)
Data Type
Reference
RMS URI/URL
Not applicable
Value(s) Reference to the relevant ActivityDefinition resource describing the
manufacturing business operation.
ISO Element Name
Manufacturer(Organisation)
ISO Path MedicinalProduct/PackagedMedicinalProduct/ManufacturerEstablishmentOr
ganisation/Manufacturer(Organisation)
FHIR Element Name
manufacturer
FHIR Path DeviceDefinition.manufacturerReference.extension.manufacturingBusiness
Operation
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.11. Manufactured item
The product as it is authorised and, where applicable, before transformation into the administrable
pharmaceutical form shall be described in this section. There after referred to as the manufactured
item, as contained in the packaged medicinal product.
A Medicinal Product may contain, in the packaging, one or more manufactured items and
corresponding to one or more pharmaceutical products.
Examples of single manufactured item and single pharmaceutical product:
• “Film-coated tablet” involves a single manufactured item. This manufactured item corresponds with
the administrable dose form and pharmaceutical product (no previous preparation/combination
with other manufactured item is needed).
• Solution for Injection involves a single manufactured item. This manufactured item corresponds
with the administrable dose form and pharmaceutical product (previous preparation/combination

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 168/231
with other manufactured item is needed to prepare the administrable dose form, however the
medicinal product does not include the solvent).
Examples of multiple manufactured items and single pharmaceutical products:
• Powder for solution for injection and Solvent for Solution for injection in combined pharmaceutical
form Powder and Solvent for Solution for injection. This involves two manufactured items that
should be combined to prepare the administrable dose form and pharmaceutical product.
Examples of multiple manufactured items and multiple pharmaceutical products:
• “Oral Capsule” & “External Cream” correspond with two different administrable dose forms which
does not need combining for administration to the patient.
The full information on Manufactured Item as presented in the FHIR ManufacturedItemDefinition is
shown in the figure 19
below. Only the elements described below are within the scope of iteration 1
implementation:
Figure 29: ManufacturedItemDefinition captured by FHIR (source: http://www.hl7.org/fhir/)
The Manufactured item class is mandatory and repeatable while the individual attributes of this class
are not repeatable (except for Ingredient class of data elements) and shall be populated as applicable.
Manufactured item Class Description
Repeatable
Yes
Conformance
Mandatory
4.11.1. Unit of presentation
Tag Description
User Guidance The unit of presentation describing the unit in which a manufactured item
is presented to describe the strength or quantity shall be specified as a
term ID.
The applicable value shall be selected from the term ID listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000000014
Value(s) As listed in the Units of Presentation RMS list, or reference for externally
maintained list in order to allow international information exchange.
ISO Element Name
Unit of Presentation

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 169/231
Tag Description
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Man
ufacturedItem/UnitOfPresentation
FHIR Element Name
unitOfPresentation
FHIR Path
ManufacturedItemDefinition.unitOfPresentation
Example(s):
- units of presentation: actuation, patch, tablet
4.11.2. Manufactured item quantity
Tag Description
User Guidance The quantity (or count number) of the manufactured item in the medicinal
product package, shall be specified as a value and units as per section 6.5
of the SmPC.
For solid dose forms and other items measured by counting (i.e., tablets,
capsules), discrete countable entities, the unit for quantity is the term
“countable unit” from the RMS Units of Measurements List and the “unit of
presentation” is the item counted within the immediate container to be
populated in the data element 4.10.1 Unit of presentation.
For formulations contained in a vial, the unit for quantity is
volume/quantity expressed with the relevant units of measurement term
(i.e., mg, mL) and the “unit of presentation” is the discrete countable
entity, in which a pharmaceutical product or manufactured item is
presented. This last data to be reported in the data element 4.10.1. Unit of
presentation.
Example:
• In case of tablets/capsules the number of tablet/capsules in the
immediate shall be specified: 28 tablets, 24 capsules are to be is to be
populated as 28 and 24 countable unit(s) respectively. The UoP
“tablets” and “capsules” are to be included in 4.10.1. respectively.
• In case of formulations contained in a vial (e.g., liquids) the total
quantity/volume should be expressed: 5 mg, 2 mL are to be populated
as 5 ml and 2 mL respectively. The UoP “vial” is to be included in
4.10.1.
• In case of lyophilised formulations contained in a vial (e.g., powder),
the total quantity/volume should be expressed: 1 vial is to be
populated as 1 countable unit(s). The UoP “vial” is to be included in
4.10.1.
For the purpose of data entry, it is recommended that this information is
specified in the SmPC of the medicinal product as much complete as
possible.
Repeatable
No
Conformance
Conditional

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 170/231
Tag Description
Data Type
Quantity
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000110633
Value(s) Numeric value and unit.
The units shall be specified as a Term ID listed in the Units of
Measurement RMS list as applicable.
ISO Element Name
Manufactured Item Quantity
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Man
ufacturedItem/ManufacturedItemQuantity
FHIR Element Name
amountQuantity
FHIR Path
PackagedProductDefinition.package.containedItem.amountQuantity
Example(s):
- 28 dispersible tablets is:
Manufactured item
Unit of presentation
Tablet
Manufactured item quantity
28 countable unit(s)
Manufactured dose form
Dispersible tablet
- 25 ml in a syringe is:
Manufactured item
Unit of presentation
Syringe
Manufactured item quantity
25 ml
Manufactured dose form
Solution for injection
4.11.2.1. Quantity operator (New)
Tag Description
User Guidance The applicable value corresponding to the quantity operator shall be
specified as term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000008
Value(s) As listed in the Quantity Operator RMS list. If the RMS term can be
mapped to a FHIR Quantity comparator, the FHIR comparator field should
also be specified for interoperability.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Quantity Operator

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 171/231
Tag Description
FHIR Path PackagedProductDefinition.package.containedItem.amountQuantity.extensi
on.quantityOperator
PackagedProductDefinition.package.containedItem.amountQuantity.compa
rator
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.11.3. Manufactured dose form
The manufactured dose form corresponds with the dose form presented in the manufactured item. See
the following examples:
Example 1:
Medicinal Product ABC 20mg/ml powder and solvent for solution for injection (combined
pharmaceutical form) in a vial will contain two types of manufactured items with the following dose
forms:
• Powder for solution for injection
• Solvent for Solution for injection
Example 2:
Medicinal Product DEF 500 mg tablets contain a single type of manufactured item with the following
manufactured dose form:
• Tablet
Tag Description
User Guidance The manufactured dose form described with the authorised pharmaceutical
form(s) in section 3. Pharmaceutical Form of the SmPC or other regulatory
document (description prior to any transformation into the final form
administered to the patient) shall be specified as a term ID.
• The required authorised pharmaceutical form shall be specified as a
term ID as listed in the applicable Referentials Management Service
(RMS) list.
• If multiple values apply to the same medicinal product, then multiple
manufactured items shall be created.
• Deprecated (i.e., non-current) dose form terms may be referenced.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000000004
Value(s)
Listed in the Pharmaceutical Dose Form RMS list.
ISO Element Name
Manufactured Dose Form

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 172/231
Tag Description
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Man
ufacturedItem/ManufacturedDoseForm
FHIR Element Name
ManufacturedDoseForm
FHIR Path
ManufacturedItemDefinition.manufacturedDoseForm
Example(s):
Manufactured pharmaceutical forms identical to the administrable pharmaceutical form: solution for
injection, tablet, capsule, inhalation powder.
Manufactured pharmaceutical forms not identical to the administrable pharmaceutical form: powder
and solvent for solution for injection, gel in sachet, syrup in sachet, emulsion for injection/infusion in
pre-filled syringe.
The manufactured dose form will be chosen only from the RMS list Pharmaceutical Dose Form and not
from the other three RMS lists that are also used for the Authorised Dose Form (Combined
pharmaceutical dose form, Combined terms or Combination Packs, section 1.5 (Authorised)
pharmaceutical form).
4.11.4. Ingredient
The ingredient(s) of a manufactured item shall be specified by selecting the relevant ingredients
present in the manufactured item, from the Ingredients lists based on the Resource Ingredient as
outlined in section 5. Ingredient.
The Ingredient class is mandatory. For further details relating to technical information and business
rules, please refer to section 5. Ingredient.
This value is an attribute within Packaged Product domain.
Tag Description
User Guidance The reference to the ingredient(s) of the pharmaceutical product shall be
selected from the list of ingredients recorded in sections 5. Ingredient
Repeatable
Yes
Conformance
Mandatory
Data Type
Reference
RMS URI/URL
Not applicable
Value(s) Structured data elements referencing the included ingredients in the
manufactured item
ISO Element Name
Ingredient
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient
FHIR Element Name
Ingredient
FHIR Path
ManufacturedItemDefinition.ingredient

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 173/231
4.11.5. Manufactured item description
The description of manufactured item shall be provided whenever the medicinal product contains more
than one manufactured item product in order to allow a readily differentiation in the database.
The provision of manufactured item characteristics is not needed for medicinal products with a single
manufactured item.
In medicinal products containing more than one manufactured item (e.g., contraceptive having
different strengths and fixed dose combination as part of the same medicinal product), differentiation
of manufacturing items is not easily visualised with the information provided in the rest of individual
data fields (in certain cases it can only be differentiated on specific substance of the qualitative and
quantitative composition of the pharmaceutical product).
Information on manufacturing item description is free text and should be provided in the form of:
“Physical Characteristics” + “Unit of presentation”.
Physical Characteristics of the manufacturing item includes colour or shape among others which allow
differentiation from remaining manufacturing items.
Note: Additional clarifications on the manufacturing item description will be provided in the EU IG v2.2
release.
Tag Description
User Guidance
The high-level description of manufactured item shall be provided whenever
the medicinal product contains more than one pharmaceutical product (e.g.,
different types of tablets in the same package).
Information on manufacturing item description is free text and should be
provided in the form of: “Physical Characteristics” + “Manufactured dose form”
+ “Package item container (immediate packaging)” as relevant.
Products authorised through MRP/DCP/NP routes
• The manufactured item description is to be provided in English or in the
local language(s) of authorisation, or optionally in all of them.
Products authorised through the centralised procedure
• The manufactured item description is to be provided in English.
Repeatable
Yes
Conformance
Conditional
Data Type
Markdown
RMS URI/URL
Not applicable
Value(s)
Free text or markdown text for rich content
ISO Element
name
Not Applicable
ISO Path
Not Applicable
FHIR Element
name
text
FHIR Path
ManufacturedItemDefinition.property.valueCodeableConcept.text
Example(s):

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 174/231
Dark yellow film-coated tablet to differentiate from Medium red film-coated tablet
4.11.5.1. Language
This section described how to populate information related to the language of the manufactured item
description. The provision of the language is mandatory.
Tag Description
User Guidance The language of the manufactured item description, as approved by the
regulatory authority and indicated in the corresponding regulatory
document(s) shall be specified as a term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072057
Value(s)
As indicated in the Language RMS list
ISO Element Name
Not Applicable
ISO Path
Not Applicable
FHIR Element Name
language
FHIR Path ManufacturedItemDefinition.property.valueCodeableConcept.text.extension
.language
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
4.12. Shelf life / Storage
The description of the shelf life and storage information of the packaged medicinal product, as
approved in the terms of the marketing authorisation, should be specified. The shelf life and storage
conditions/scenarios should be listed in the Product information and section 2.2.3 of the electronic
application form (eAF). This includes shelf life and storage conditions as packaged for sale, shelf
life/storage conditions after dilution or reconstitution or any other scenario.
This entity is repeatable to allow the introduction of different shelf-life/storage conditions in the same
product (e.g., shelf life and storage conditions for medicinal products as packaged for sale and after
dilution or reconstitution). An example is shown below:
Example(s):
Packaged Medicinal Product
Package description
Type I glass vial with rubber stopper containing 100 mg of
active substance abc. Pack of 1 vial.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 175/231
Description (as per SmPC) Unopened vial
3 years.
Do not store above 30
o
C
Reconstituted and infusion solutions
When prepared as directed, reconstituted and infusion
solutions of Medicine ABC contain no antimicrobial
preservatives. Chemical and physical in-use stability of
reconstituted and infusion solutions of Medicine ABC were
demonstrated for 24 hours at refrigerated temperature.
From a microbiological point of view, the product should be
used immediately. If not used immediately, in-use storage
times and conditions prior to use are the responsibility of the
user and would not be longer than 24 hours at 2°C to 8°C.
Shelf life type (1) Shelf life of the medicinal product as packaged for sale
Shelf life time and Period (1) 3 years
Storage Conditions (1) Do not store above 30
o
C
Shelf life type (2) Shelf life after dilution or reconstitution according to directions
Shelf life time and Period (2) 24 hours
Storage Conditions (2) Store in a refrigerator (2ºC – 8ºC)
Shelf Life/ Storage Condition should be linked to the Package Item Container to representing the
overall packaged medicinal product (outer-most package item) unless different shelf-life and storage
conditions for different packaged items are specified in sections 6.3 – Shelf Life and 6.4 - Special
precautions for storage of the relevant SmPC. In this case the Shelf Life/ Storage Conditions should be
linked to the most relevant Package Item Container.
The full information on Storage / Shelf Life is shown in the figure 20 below. Only the elements inside
the red rectangle apply for the iteration 1 implementation.
Figure 30: Shelf Life / Storage as captured by FHIR (source: http://www.hl7.org/fhir/)
The Shelf life/storage class is conditional and repeatable while the individual attributes of this class are
not repeatable (with the exception of special precaution for storage data element) and are mandatory
to be populated.
Shelf life/storage Class Description
Repeatable
Yes

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 176/231
Shelf life/storage Class Description
Conformance
Conditional
4.12.1. Shelf life type
Tag Description
User Guidance
The type of the shelf life such as the shelf life applicable to the whole
Packaged Medicinal Product itself, or more granular values such as the
shelf-life after transformation, shelf life after the initial opening of a bottle
or any other scenario covered in the product information, shall be specified
as a term ID from the Shelf Life Type List in the Referentials Management
Service (RMS) list.
This information is to be completed as per section 6.3 – Shelf life of the
SmPC and section 2.2.3 of the electronic application form (eAF). This field
is repeatable to cover multiple different Shelf-Life conditions.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL https://spor.ema.europa.eu/v1/lists/100000073343
Value(s)
As listed in the Shelf Life Type RMS list
ISO Element name
Shelf Life Type
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Shelf
Life-Storage/ShelfLifeType
FHIR Element name
Type
FHIR Path
PackagedProductDefinition.package.shelfLifeStorage.type
Example(s):
Shelf life of the medicinal product as packaged for sale.
Shelf life after first opening the immediate packaging.
4.12.2. Shelf life time period and units
Tag Description
User Guidance The shelf life time period shall be specified using a (1) numerical value for
the period and (2) its unit of time measurement. Multiple shelf life periods
may be listed for different types.
This information is to be completed as per section 6.3 – Shelf life of the
SmPC and section 2.2.3 of the electronic application form (eAF). This field
is repeatable to cover multiple different Shelf-Life conditions.
This field is an attribute within Shelf Life Type.
Repeatable
No
Conformance
Mandatory
Data Type
Quantity

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 177/231
Tag Description
RMS URI/URL https://spor.ema.europa.eu/v1/lists/100000110633
Value(s) Value is a numerical value with implicit precision and should be reflected
accordingly
The units shall be specified as a Term ID as listed in the Units of
Measurement RMS list
ISO Element name
Shelf Life Time Period
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Shelf
Life-Storage/ShelfLifeTimePeriod
FHIR Element name
Period
FHIR Path
PackagedProductDefinition.package.shelfLifeStorage.period
Example(s):
Value Unit
5 Year(s)
12 Month(s)
24 Hour(s)
4.12.3. Special precautions for storage
Tag Description
User Guidance Special precautions for storage of the relevant Package Item Container of
the packaged medicinal product should be specified using the appropriate
value(s). The controlled term and the controlled term identifier shall be
specified.
The term “This medicinal product does not require any special storage
condition” shall be selected if no special precautions for storage apply to
the packaged medicinal product.
This information is to be completed as per section 6.4 – Special
precautions for storage of the SmPC and section 2.2.3 of the electronic
application form (eAF). This field is repeatable to cover different Storage
Conditions per Shelf life.
This field is an attribute within Shelf Life Type.
Repeatable
Yes
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000073344
Value(s)
As listed in the Special Precaution for Storage RMS list
ISO Element name
Storage.SpecialPrecautionsforStorage
ISO Path /MedicinalProduct/PackagedMedicinalProduct/PackageItem_Container/Shelf
Life-Storage/SpecialPrecautionsForStorage

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 178/231
Tag Description
FHIR Element name
specialPrecautionsForStorage
FHIR Path PackagedProductDefinition.package.shelfLifeStorage.specialPrecautionsFor
Storage
Example(s):
Do not store above 25 °C
Do not store above 30 °C
Do not freeze
Store in a refrigerator (2°C - 8°C)
Store in the original package in order to protect from light

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 179/231
5. Ingredient
The full information on ingredient(s) of a manufactured item and of the pharmaceutical product is
described by the FHIR Resource Ingredient as shown in figure 21 below. Note that when describing
ingredients of manufactured item (section 4.11.4) and pharmaceutical product (section 6.4), applicable
ingredients shall be selected from the list of ingredients relevant to the medicinal product as described
in this section. Also, the same ingredient can be referenced in both the manufactured item and
pharmaceutical product, when needed. The class is mandatory. In the context of the Iteration 1 of the
PMS implementation, the following elements are in scope and information should be provided
according to the rules and guidance described in this section.
Figure 31: Resource Ingredient (Source : http://www.hl7.org/fhir)
The entire Ingredient class is mandatory and repeatable while the individual attributes of this class are
not repeatable and shall be populated as applicable.
Ingredient Class Description
Repeatable
Yes
Conformance Mandatory
Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 180/231
5.1. Ingredient role
Tag Description
User Guidance The role of the ingredient as part of the manufactured item/pharmaceutical
product shall be specified as a term ID.
The applicable value(s) shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072050
Value(s)
As listed in the Ingredient Role RMS list
ISO Element Name
Ingredient Role
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/IngredientRole
For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient/IngredientRole
FHIR Element Name
Role
FHIR Path
Ingredient.role
Example(s):
Active, excipient, adjuvant
5.2. Origin of the substance
Tag Description
User Guidance
The origin of the source material of the substance can be specified.
Repeatable
No
Conformance
Optional
Data Type
CodeableConcept
RMS URI/URL
To be created
Value(s) The applicable value as listed from the RMS list [list will be created in
v2.2]
ISO Element Name Not applicable
ISO Path
Not applicable
FHIR Element Name
originOfSubstance
FHIR Path Ingredient.extension.originOfSubstance
Example:
Animal Origin susceptible to TSE; Other Animal Origin; Human Origin

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 181/231
5.3. Composition grouping description
Tag Description
User Guidance Information to specify to which part of the manufactured item each
ingredient belongs to (i.e., coating, printing ink) can be reported as free
text.
Repeatable
No
Conformance
Optional
Data Type
CodeableConcept.text
RMS URI/URL
Not applicable
Value(s)
The composition grouping description as free text
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
group
FHIR Path Ingredient.extension.group
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
5.4. Manufacturer
The manufacturer of the active substance (including active substance intermediate manufacturers) and
the adjuvant for combined pharmaceutical forms, shall be specified from the list of manufacturers
based on the resource MedicinalProductDefinition.manufacturingBusinessOperation.manufacturer as
outlined in section 1.20.1.
The class is conditional, provided that ingredient role specified is the Active Substance or Adjuvant. For
further details relating to technical information and business rules, please refer to section 1.20.
Manufacturing business operation.
Manufacturers of active substance intermediates shall also be selected and associated to the Active
Substance. The manufacturing sites performing the following operations are associated to substances
and Adjuvants (if applicable):
• Manufacturing of the active substance (including active substance intermediates) product as
reflected in module 3.2.S.2.1 and section 2.5 of the Initial Marketing Authorisation (initial MA)
electronic Application Form (eAF).
• Manufacturer of the adjuvant as reflected in module 3.2.P.3.1 and section 2.5 of the Initial
Marketing Authorisation (initial MA) electronic Application Form (eAF).
• The reference to the ingredient(s) of the pharmaceutical product shall be selected based on the list
of ingredients recorded in sections 5. Ingredient
Tag Description
User Guidance
The reference to the relevant Manufacturer of the active substance
(including active substance intermediate manufacturers) and the adjuvant
for combined pharmaceutical forms shall be selected from the list of
manufacturers recorded in section 1.20 Manufacturing business operation.
Repeatable
Yes
Conformance
Conditional

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 182/231
Tag Description
Data Type
Reference
RMS URI/URL
Not applicable
Value(s) Reference to the relevant ActivityDefinition resource describing the
manufacturing business operation.
ISO Element Name
Manufacturer(Organisation)
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/Manufacturer(Organisation)
For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient/
Manufacturer(Organisation)
FHIR Element Name
manufacturer
FHIR Path Ingredient.manufacturer.extension.manufacturingBusinessOperation
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
5.5. Substance
The entire Substance sub-class is mandatory and not repeatable while the individual attributes shall be
repeated and populated as applicable.
Ingredient Class Description
Repeatable
No
Conformance
Mandatory
5.5.1. Substance
Section 2. Qualitative and Quantitative composition of the SmPC, section 6.1. List of excipients of the
SmPC and Module 3.2.P.1 – Description and Composition of the Drug Product indicate the composition
of pharmaceutical product(s) within the medicinal product.
Each pharmaceutical product or Manufactured Item shall contain information on:
• Active ingredient(s) - active ingredient substance name(s). For Pharmaceutical Product, the active
ingredient(s) can be found in Section 2. Qualitative and Quantitative composition of the SmPC and
Module 3.2.P.1 of the dossier.
• Excipient(s) - excipient substance name(s). For Pharmaceutical Product, the excipient(s) can be
found in Module 3.2.P.1 of the dossier and Section 6.1. List of excipients of the SmPC.
• In some instances, the composition of the medicinal product can also contain adjuvants. Adjuvant
substance name(s) can be found in Section 2. Qualitative and Quantitative composition of the
SmPC and Module 3.2.P.1 of the dossier.
The Substance(s) contained in pharmaceutical product or Manufactured Item shall be specified as a
term ID.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 183/231
If the required substance term and related identifier is not available, the addition of the unlisted term
ID should be requested from SMS via EMA Service Desk portal.
Note 1: Substance Management Service (SMS) will release in future the guidance describing the
process to submit substance change request(s) in SMS.
Note 2: every pharmaceutical product shall have at least one ingredient, regardless the ingredient role.
If a pharmaceutical product contains no active ingredient, the excipients should be labelled as excipient
and not as active ingredient (example: water for injection as a solvent in a separate container, glucose
solution 5%, NaCl solution 0.9%.; contraceptive tablets containing only lactose). This rule differs from
the existing xEVMPD business rule.
Tag Description
User Guidance The Substances contained within the medicinal product (either part of the
pharmaceutical product(s) or the manufactured item(s)) shall be specified.
Each pharmaceutical product or Manufactured Item shall contain
information on:
• active ingredient(s);
• excipient(s);
• in some instances, pharmaceutical product can also contain adjuvants.
The Substance(s) contained in pharmaceutical product or Manufactured
Item shall be specified as a term ID.
The selected SMS ID refers to a particular substance, and the preferred
term (PT) for that substance will always be the name displayed in PMS. In
many cases, alternate names are stored for substances and the SMS ID
can be found by using any of the names associated with a substance.
Note: every pharmaceutical product shall have at least one ingredient. If a
pharmaceutical product contains no active ingredient, the excipients shall
be labelled as excipient.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
Not applicable
Value(s)
As listed in SPOR Substance Management System (SMS ID)
ISO Element Name
Substance
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/Substance
For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient/Substance
FHIR Element Name
codeCodeableConcept
FHIR Path
Ingredient.substance.codeCodeableConcept

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 184/231
5.5.2. Substance strength (quantitative composition)
The Strength (quantitative composition) of the substance (active substances, excipients and
adjuvants) should be declared as a quantity of the substance contained in a Manufactured Item or
Pharmaceutical Product.
The strength (quantitative composition) shall be provided based on a numerator and denominator
value and unit, i.e., unit of presentation or unit of measure/concentration.
When the strength of a medicinal product described as a technical concept of a "Pharmaceutical
Product" that has undergone a transformation (for example reconstitution) is to be specified, it is to be
specified using the strength resulting from transformation undertaken exactly in accordance with the
regulated product information (i.e., in the SmPC as per Section 2. Qualitative and Quantitative
Composition and Module 3.2.P.1 of the dossier). This information can also be found in other parts of
the SmPc. No calculations/conversions should be performed to obtain a figure.
Where the active ingredient is an ester or pro-drug, the quantitative composition shall be stated in
terms of the quantity of that ester or pro-drug. In this case, the strength of the active moiety should
be entered in the reference strength section (refer to section 5.5.3.
Substance reference strength
(quantitative composition)). The following example illustrates this requirement:
Example(s):
Ingredient active substance contains a derivative
Field Value
SmPC text
Each tablet contains 12 mg loperamide
hydrochloride corresponding to 10 mg
loperamide
Active substance loperamide hydrochloride
Active substance strength presentation single
value or low limit numerator
12 mg
Active substance strength presentation single
value or low limit denominator
1 tablet
Reference substance Loperamide
Reference substance strength presentation single
value or low limit numerator
10 mg
Reference substance strength presentation single
value or low limit denominator
1 tablet
Expression of the strength (quantitative composition)
The strength (quantitative composition) can be expressed as:
• Presentation strength (per unit of presentation) - strength expressed per unit of
presentation.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 185/231
The unit of presentation is a qualitative term describing the discrete unit in which a Pharmaceutical
Product is presented.
Example:
250 milligrams per tablet
10 mg per vial (for solution for injection)
120 mg per bottle (for powders or granules for liquid preparation)
10 mg per tube (for single use gels)
54 microgram per actuation (for inhaler with individual capsules, delivered dose shall be used)
• Concentration strength (per unit of measure/concentration) – the strength is expressed as
the amount of the substance per unit of measurement such as millilitre or gram (not unit of
presentation)
The concentration strength is always expressed per single unit of measurement, e.g., 4 mg/1 ml. A
strength expressed as e.g., 4 mg/0.8 ml is a presentation strength since the 0.8 ml is the amount
of the solution in the presentation. The concentration strength equivalent to that would be 5 mg/1
ml.
Example:
10 milligrams per 1 millilitre (for parenteral products)
10 milligrams per 24 hours (for transdermal patches)
100 countable units per 1 millilitre (for insulins)
The provision of the strength(s) of the active ingredient(s) is mandatory. The strength of the active
substance as listed in section 2. Qualitative and Quantitative Composition of the corresponding SmPC
and in Module 3.2.P.1 of the dossier shall be specified. For further information related on the
reporting of composition, refer to section 3.3 Expression of strength of EU IG Chapter 8.
Overall, when expressing the strength of active ingredients, applicants and marketing authorisation
holders should abide to the following principles:
• The SmPC should be used as a main reference for expressing the strength in ingredients to be
linked both to the manufactured item and pharmaceutical product.
• The quantitative composition of the active ingredient should be expressed by means of
Presentation strength and depending on cases Concentration strength. If the presentation strength
is clearly stated in the relevant SmPC and/or Module 3, it shall be populated in addition to the
concentration strength if both ways to express the strength are included in the SmPC/Module 3.
• Chapter 8 – Practical examples and QRD guidance on expression of strength provides guidance on
how to express the strength per type of medicinal products.
• The expression of the strength in the SmPC has been approved by a regulatory scientific
assessment and therefore should be used in all cases as the main reference to express the
strength. In case of difference on how the strength is expressed for a specific medicinal product in
the SmPC and the guidance provided in Chapter 8 – Practical examples and QRD guidance on
expression of strength, the information on the SmPC prevails.
• Where the active ingredient is an ester or pro-drug, the quantitative composition shall follow how
the substance is expressed in the SmPC. SmPC guidance says that the substance should be stated

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 186/231
in terms of the quantity of that ester or pro-drug. If the active moiety is an active substance in an
already approved medicinal product, the reference substance should be given in terms of this
active moiety (as per the SmPC guidelines, e.g., 75 mg of fosphenytoin is equivalent to 50 mg of
phenytoin). If the active moiety has not been approved separately, then the reference substance
should be the ester or pro-drug and not any presumed active moiety. See chapter 2, section 5.5.3
Substance reference strength (quantitative composition).
If the product contains excipient(s) and/or adjuvant(s), the provision of the amount(s) of the
ingredient(s) shall be specified.
In cases where the quantitative composition of the excipient(s) and/or adjuvant(s) of the medicinal
product is not reported in the relevant product information (i.e., SmPC), the substance strength is to
be left empty and the substance reference strength is to be completed. For further information on this
exceptional case (i.e., vaccines) refer to Chapter 8 – Practical example.
The strength of the excipient(s) and adjuvant(s) as listed in section 2. Qualitative and Quantitative
Composition (or in section 6. List of excipients) of the corresponding SmPC and in Module 3.2.P.1 of
the dossier shall be specified.
The entire Substance Strength (quantitative composition) sub-class is conditional and not repeatable
while the individual attributes of this sub-class are not repeatable and shall be populated as applicable.
Ingredient Class Description
Repeatable
No
Conformance
Conditional
5.5.2.1. Quantity operator
The Strength (quantitative composition) of the substance (active substances, excipients and
adjuvants) should be declared as a quantity of the substance contained in a Manufactured Item or
Pharmaceutical Product [for further details refer to section 5.5.2. Substance strength (quantitative
composition)and192Substance reference strength (quantitative composition)].
To precisely express the strength of the substance ingredient(s) contained in the medicinal product,
the corresponding quantity operator shall be selected.
The quantity operator applies to each of the below data elements based:
• Strength (Presentation) 5.5.2.2.1. Quantity operator
• Strength (Concentration) 5.5.2.3.1. Quantity operator
For further information on the technical details of this data element, refer to the below sections
5.5.2.2.1. and 5.5.2.3.1.
Example(s):
Equal to, less than, more than etc.
5.5.2.2. Strength (presentation)
When the strength of a substance is described as a qualitative term describing the discrete unit, the
below fields should be used to enter this information.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 187/231
The entire Strength (presentation) sub-class is conditional and not repeatable while the individual
attributes of this sub-class are not repeatable and shall be populated as applicable.
Ingredient Class Description
Repeatable
No
Conformance
Conditional
Note: If the Strength (presentation) is clearly stated in the relevant SmPC and/or Module 3, this field
shall be populated. For further information, refer to the table reported in section 3.3. Expression of
strength in Chapter 8 – Practical example.
5.5.2.2.1. Quantity operator
Tag Description
User Guidance The applicable value corresponding to the quantity operator shall be
specified as term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000008
Value(s) As listed in the Quantity Operator RMS list. If the RMS term can be
mapped to a FHIR Quantity comparator, the FHIR comparator field should
also be specified for interoperability.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name quantityOperator
FHIR Path Ingredient.substance.strength.extension.quantityOperator
Ingredient.substance.strength.presentation.numerator.comparato
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
5.5.2.2.2. Strength (presentation single value or low limit)
Tag Description
User Guidance
The strength (quantitative composition) of the substances (including active
substances, ingredients, adjuvant as applicable) shall be specified in this
field with a numerator and denominator.
When the strength is expressed as a range, the lower limit for the quantity
of the substance in the unit of presentation shall be specified in this field.
When the strength is not expressed as a range, the quantity of the
substance shall be specified in this field.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 188/231
Tag Description
The numerator should be expressed with a unit (numeric value) and a unit
of measurement (e.g., mg).
The denominator should be expressed with a unit (numeric value) and a
unit of presentation (e.g., tablet, actuation).
Repeatable
No
Conformance
Mandatory
Data Type
Ratio
RMS URI/URL • https://spor.ema.europa.eu/v1/lists/100000110633
•
https://spor.ema.europa.eu/v1/lists/200000000014
Value(s) The units for the numerator shall be specified as a value and a Term ID as
listed in the Units of Measurement RMS list.
The units for the denominator shall be specified as a value and a Term ID
as listed in the Units of Presentation or the Units of Measurement RMS
list.
ISO Element Name
Strength (Presentation)
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/Substance/Strength/Strength_Presentation
For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient/Substance/Strength/
Strength_Presentation
FHIR Element Name
Presentation
FHIR Path
Ingredient.substance.strength.presentation
5.5.2.2.3. Strength (presentation high limit)
Tag Description
User Guidance
The strength (quantitative composition) of the substances shall be
specified in this field with a numerator and denominator, only applicable
when the strength is expressed as a range.
In this case, the upper limit for the quantity of the substance in the unit of
presentation shall be specified in this field.
When the strength is not expressed as a range, this field shall not be
populated.
The numerator should be expressed with a unit of numeric value and a
unit of measurement (e.g., mg).
The denominator should be expressed with a unit of numeric value and a
unit of presentation (e.g., tablet).
Repeatable
No
Conformance
Conditional
Data Type
Ratio
RMS URI/URL • https://spor.ema.europa.eu/v1/lists/100000110633
•
https://spor.ema.europa.eu/v1/lists/200000000014

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 189/231
Tag Description
Value(s) The units for the numerator shall be specified as a value and a Term ID as
listed in the Units of Measurement RMS list.
The units for the denominator shall be specified as a value and a Term ID
as listed in the Units of Presentation or the Units of Measurement RMS list.
ISO Element Name
Strength (Presentation)
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/Substance/Strength/Strength_Presentation
For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient/Substance/Strength/
Strength_Presentation
FHIR Element Name
PresentationHighLimit
FHIR Path
Ingredient.substance.strength.presentationHighLimit
Example(s):
Tablet 500 mg
Strength presentation single value or low limit: numerator 500 mg, denominator 1 tablet
Strength presentation high limit: <blank>
Powder for solution for injection 88-90 mg in a vial
Strength presentation single value or low limit: numerator 88 mg, denominator 1 vial
Strength presentation high limit: 90 mg, denominator 1 vial
5.5.2.3. Strength (concentration)
When the strength of a substance is expressed as the amount of substance per unit of measurement,
such as millilitre or gram, the below fields should be used to enter this information.
The entire Strength (concentration) sub-class is conditional and not repeatable while the individual
attributes of this sub-class are not repeatable and shall be populated as applicable.
Ingredient Class Description
Repeatable
No
Conformance
Conditional

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 190/231
5.5.2.3.1. Quantity operator
Tag Description
User Guidance
The applicable value corresponding to the quantity operator shall be
specified as term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000008
Value(s) As listed in the Quantity Operator RMS list. If the RMS term can be
mapped to a FHIR Quantiy comparator, the FHIR comparator field should
also be specified for interoperability.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
quantityOperator
FHIR Path Ingredient.substance.strength.extension.quantityOperator
Ingredient.substance.strength.concentration.numerator.comparator
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
5.5.2.3.2. Strength (concentration single value or low limit)
Tag Description
User Guidance The strength (quantitative composition) of the substances shall be
specified in this field with a numerator and denominator.
When the strength is expressed as a range, the lower limit for the quantity
of the substance in the unit of measurement shall be specified in this field.
When the strength is not expressed as a range, the quantity of the
substance shall be specified in this field.
The numerator and the denominator should be expressed with a unit of
numeric value and a unit of measurement (e.g., mg, ml).
Repeatable
No
Conformance
Mandatory
Data Type
Ratio
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000110633
Value(s) The units for the numerator and the denominator shall be specified as a
value and a Term ID as listed in the Units of Measurement RMS list.
ISO Element Name
Strength (Concentration)
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/Substance/Strength/Strength_Concentration
For the Pharmaceutical product, the ISO path is:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 191/231
Tag Description
/MedicinalProduct/PharmaceuticalProduct/Ingredient/Substance/Strength/
Strength_Concentration
FHIR Element Name
Concentration
FHIR Path
Ingredient.substance.strength.concentration
5.5.2.3.3. Strength (concentration high limit)
Tag Description
User Guidance The strength (quantitative composition) of the substances shall be
specified in this field with a numerator and denominator, only applicable
when the strength is expressed as a range.
In this case, the upper limit for the quantity of the substance in the unit of
measurement shall be specified in this field.
When the strength is not expressed as a range, this field shall not be
populated.
The numerator and the denominator should be expressed with a unit of
numeric value and a unit of measurement (e.g., mg, ml).
Repeatable
No
Conformance
Conditional
Data Type
Ratio
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000110633
Value(s) The units for the numerator and the denominator shall be specified as a
value and a Term ID as listed in the Units of Measurement RMS list.
ISO Element Name
Strength (Concentration)
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/Substance/Strength/Strength_Concentration
For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient/Substance/Strength/
Strength_Concentration
FHIR Element Name
ConcentrationHighLimit
FHIR Path
Ingredient.substance.strength.concentrationHighLimit
Example(s):
Solution for injection 20 mg/ml
Strength concentration single value or low limit: numerator 20 mg, denominator 1 ml
Strength concentration high limit: <blank>
Powder and solvent for solution for injection 95-100 mg/ml
Strength concentration single value or low limit: numerator 95 mg, denominator 1 ml
Strength concentration high limit: 100 mg, denominator 1 ml

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 192/231
5.5.3. Substance reference strength (quantitative composition)
Reference strength represents the strength (quantitative composition) of the active moiety of the
active substance or of another substance used to express the strength of the product. There are
situations when the active substance and active moiety are different resulting in different expression of
the strength.
For example, if an active substance is in the form of a salt or hydrate, the reference strength shall be
expressed in terms of the mass [or biological activity in International (or other) units where
appropriate] of the active moiety (base, acid or anhydrous material).
The reference substance and reference strength of the active substance(s) contained in the
Pharmaceutical product or Manufactured Item can be found in section 2. Qualitative and Quantitative
Composition of the corresponding SmPC.
The reference strength shall be provided for active substances and is repeatable. However, there are
cases where the reference strength of the active substance may not be provided. For information about
the special cases, please refer to the diagram available in section 3.3. Express strength of Chapter
8 – Practical example.
In cases where the quantitative composition of the excipient(s) and/or adjuvant(s) of the medicinal
product is not reported in the relevant product information (i.e., SmPC), the substance strength is to
be left empty and the substance reference strength is to be completed.
For further information on the above exceptional cases (i.e., vaccines) refer to section 3.3. Express
strength of Chapter 8 – Practical example.
Note that the ISO standards do not explicitly differentiate between Strength (Presentation) versus
Strength (Concentration) for Reference Strengths. PMS will do that, so the ISO paths below are not
unique for these two different strength expressions.
Example(s):
Field Value
SmPC text Each tablet contains 12 mg loperamide
hydrochloride corresponding to 10 mg
loperamide
Active substance loperamide hydrochloride
Active substance strength presentation single
value or low limit numerator
12 mg
Active substance strength presentation single
value or low limit denominator
1 tablet
Reference substance Loperamide
Reference substance strength presentation single
value or low limit numerator
10 mg
Reference substance strength presentation single
value or low limit denominator
1 tablet

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 193/231
Field Value
SmPC text Containing 538.20 mg of valproate semisodium
per tablet (equivalent to 500 mg of valproic
acid).
Active substance valproate semisodium
Active substance strength presentation single
value or low limit numerator
538.20 mg
Active substance strength presentation single
value or low limit denominator
1 tablet
Reference substance valproic acid
Reference substance strength presentation single
value or low limit numerator
500 mg
Reference substance strength presentation single
value or low limit denominator
1 tablet
In addition, if the active substance is not a salt/hydrate, the strength [Section 5.5.2. Substance
strength (quantitative composition)] and reference strength in the product composition will be the
same and the strength information shall be repeated in the reference strength (reference strength is
mandatory for active substances)
Example(s):
Field Value
SmPC text
Each 0.2 ml single dose pre-filled syringe
contains 20 mg of adalimumab
Active substance Adalimumab
Active substance strength presentation single
value or low limit numerator
20 mg
Active substance strength presentation single
value or low limit denominator
0.2 ml
Reference substance Adalimumab
Reference substance strength presentation single
value or low limit numerator
20 mg
Reference substance strength presentation single
value or low limit denominator
0.2 ml
Reference substance strength concentration
single value or low limit numerator
100 mg
Reference substance strength concentration
single value or low limit denominator
1 ml

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 194/231
The entire Reference strength sub-class is conditional based on the below business rules and not
repeatable while the individual attributes of this sub-class are not repeatable and shall be populated as
applicable.
Ingredient Class Description
Repeatable
No
Conformance
Conditional
5.5.3.1. Reference substance
Tag Description
User Guidance The reference substance of the active substance(s) contained in
pharmaceutical product or Manufactured Item, as expressed in section 2.
Qualitative and Quantitative Composition of the corresponding SmPC and
in Module 3.2.P.1 of the dossier, shall be specified.
The reference substance shall be provided for active substances only.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
Not applicable
Value(s)
As listed in SPOR Substance Management System (SMS ID)
ISO Element Name
Reference Substance
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/Substance/Strength/ReferenceStrength/ReferenceSubstanc
e
For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient/Substance/Strength/
ReferenceStrength/ReferenceSubstance
FHIR Element Name
Substance
FHIR Path Ingredient.substance.strength.referenceStrength.
substanceCodeableConcept
5.5.3.2. Quantity operator
The Strength (quantitative composition) of the substance (active substances, excipients and
adjuvants) should be declared as a quantity of the substance contained in a Manufactured Item or
Pharmaceutical Product [for further details refer to section 5.5.2. Substance strength (quantitative
composition) and5.5.3. Substance reference strength (quantitative composition)].
To precisely express the strength of the substance ingredient(s) contained in the medicinal product,
the corresponding quantity operator shall be selected.
The quantity operator applies to each of the below data elements based:
• Reference strength (Presentation) 5.5.3.3.1. Quantity operator
• Reference strength (Concentration) 5.5.3.4.1. Quantity operator

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 195/231
For further information on the technical details of this data element, refer to the below sections
5.5.3.3.1. and 5.5.3.4.1.
Example(s):
Equal to, less than, more than etc.
5.5.3.3. Reference strength (Presentation)
When the reference strength of an active substance is described as a qualitative term describing the
discrete unit, the below fields shall be used to enter this information.
The entire Reference strength sub-class is conditional and not repeatable while the individual attributes
of this sub-class are not repeatable and shall be populated as applicable.
Ingredient Class Description
Repeatable
No
Conformance
Conditional
5.5.3.3.1. Quantity operator
Tag Description
User Guidance The applicable value corresponding to the quantity operator shall be
specified as term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000008
Value(s) As listed in the Quantity Operator RMS list. If the RMS term can be
mapped to a FHIR Quantiy comparator, the FHIR comparator field should
also be specified for interoperability.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name quantityOperator
FHIR Path Ingredient.substance.strength.extension.quantityOperator
Ingredient.substance.strength.referenceStrength.strength.numerator.comp
arator
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
5.5.3.3.2. Reference strength (Presentation single value or low limit)
Tag Description
User Guidance The reference strength (quantitative composition) of the active substances
shall be specified in this field with a numerator and denominator.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 196/231
Tag Description
When the reference strength is expressed as a range, the lower limit for
the quantity of the reference substance in the unit of presentation shall be
specified in this field.
When the reference strength is not expressed as a range, the quantity of
the reference substance shall be specified in this field.
The numerator shall be expressed with a unit of numeric value and a unit
of measurement (e.g., mg).
The denominator shall be expressed with a unit of numeric value and a
unit of presentation (e.g., tablet).
The reference strength shall be provided for active substances only.
Repeatable
No
Conformance Mandatory
Data Type
Ratio
RMS URI/URL • https://spor.ema.europa.eu/v1/lists/100000110633
•
https://spor.ema.europa.eu/v1/lists/200000000014
Value(s) The units for the numerator shall be specified as a value and a Term ID as
listed in the Units of Measurement RMS list.
The units for the denominator shall be specified as a value and a Term ID
as listed in the Units of Presentation or Units of Presentation or the Units of
Measurement RMS list..
ISO Element Name
ReferenceStrength
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/Substance/Strength/ReferenceStrength
For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient/Substance/Strength/
ReferenceStrength
FHIR Element Name
Strength
FHIR Path
Ingredient.substance.strength.referenceStrength.strength
FHIR Complementary
Information
referenceStrength.strength.denominator.system value should refer to the
Unit of Presentation RMS list
5.5.3.3.3. Reference strength (Presentation high limit)
Tag Description
User Guidance The reference strength (quantitative composition) of the active substances
shall be specified in this field with a numerator and denominator, only
applicable when the reference strength is expressed as a range.
In this case, the upper limit for the quantity of the reference substance in
the unit of presentation shall be specified in this field.
When the reference strength is not expressed as a range, this field shall
not be populated.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 197/231
Tag Description
The numerator should be expressed with a unit of numeric value and a
unit of measurement (e.g., mg).
The denominator should be expressed with a unit of numeric value and a
unit of presentation (e.g., tablet).
The reference strength shall be provided for active substances only.
Repeatable
No
Conformance
Conditional
Data Type
Ratio
RMS URI/URL • https://spor.ema.europa.eu/v1/lists/100000110633
•
https://spor.ema.europa.eu/v1/lists/200000000014
Value(s) The units for the numerator shall be specified as a value and a Term ID as
listed in the Units of Measurement RMS list.
The units for the denominator shall be specified as a value and a Term ID
as listed in the Units of Presentation or Units of Presentation or the Units of
Measurement RMS list.
ISO Element Name
ReferenceStrength
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/Substance/Strength/ReferenceStrength
For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient/Substance/Strength/
ReferenceStrength
FHIR Element Name
StrengthHighLimit
FHIR Path
Ingredient.substance.strength.referenceStrength.strengthHighLimit
FHIR Complementary
Information
referenceStrength.strength.denominator.system value should refer to the
Unit of Presentation RMS list
5.5.3.4. Reference strength (Concentration)
When the reference strength of an active substance is expressed as the amount of substance per unit
of measurement such as millilitre or gram, the below fields should be used to enter this information.
The entire Reference strength sub-class is conditional and not repeatable while the individual attributes
of this sub-class are not repeatable and shall be populated as applicable.
Ingredient Class Description
Repeatable
No
Conformance
Conditional
5.5.3.4.1. Quantity operator
Tag Description
User Guidance The applicable value corresponding to the quantity operator shall be
specified as term ID.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 198/231
Tag Description
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000000008
Value(s) As listed in the Quantity Operator RMS list. If the RMS term can be
mapped to a FHIR Quantity comparator, the FHIR comparator field should
also be specified for interoperability.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
quantityOperator
FHIR Path Ingredient.substance.strength.extension.quantityOperator
Ingredient.substance.strength.referenceStrength.strength.numerator.comp
arator
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
5.5.3.4.2. Reference strength (Concentration single value or low limit)
Tag Description
User Guidance The reference strength (quantitative composition) of the active substances
shall be specified in this field with a numerator and denominator.
When the reference strength is expressed as a range, the lower limit for
the quantity of the reference substance in the unit of measurement shall
be specified in this field.
When the reference strength is not expressed as a range, the quantity of
the reference substance shall be specified in this field.
The numerator and the denominator shall be expressed with a unit of
numeric value and a unit of measurement (e.g., mg, ml).
The reference strength shall be provided for active substances only.
Repeatable
No
Conformance
Mandatory
Data Type
Ratio
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000110633
Value(s) The units for the numerator and the denominator shall be specified as a
value and a Term ID as listed in the Units of Measurement RMS list.
ISO Element Name
ReferenceStrength
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/Substance/Strength/ReferenceStrength
For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient/Substance/Strength/
ReferenceStrength

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 199/231
Tag Description
FHIR Element Name
Strength
FHIR Path
Ingredient.substance.strength.referenceStrength.strength
FHIR Complementary
Information
referenceStrength.strength.denominator.system value should refer to the
Unit of Measurement RMS list
5.5.3.4.3. Reference strength (Concentration high limit)
Tag Description
User Guidance The reference strength (quantitative composition) of the active substances
shall be specified in this field with a numerator and denominator, only
applicable when the strength is expressed as a range.
In this case, the upper limit for the quantity of the reference substance in
the unit of measurement shall be specified in this field.
When the reference strength is not expressed as a range, this field shall
not be populated.
The numerator and the denominator should be expressed with a unit of
numeric value and a unit of measurement (e.g., mg, ml).
The reference strength shall be provided for active substances only.
This value is an attribute within Reference Strength.
Repeatable
No
Conformance
Conditional
Data Type
Ratio
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000110633
Value(s) The units for the numerator and the denominator shall be specified as a
value and a Term ID as listed in the Units of Measurement RMS list.
ISO Element Name
ReferenceStrength
ISO Path For the Manufactured Item, the ISO path is:
/MedicinalProduct/PackagedMedicinalProduct/PackageItem/ManufacturedIt
em/Ingredient/Substance/Strength/ReferenceStrength
For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient/Substance/Strength/
ReferenceStrength
FHIR Element Name
StrengthHighLimit
FHIR Path
Ingredient.substance.strength.referenceStrength.strengthHighLimit
FHIR Complementary
Information
referenceStrength.strength.denominator.system value should refer to the
Unit of Measurement RMS list
5.5.4.
(Certificate) master file
The (Certificate) master file should be used to include the following certificates referring to the
composition of the medicinal product:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 200/231
• Certificate of suitability for Transmissible Spongiform Encephalopathies (TSEs CEP). The
TSE Certificates of suitability applies to medicinal products containing or using in the
manufacturing process materials of animal and/or human origin with a risk of transmissible
spongiform encephalopathy (TSE) in accordance with Directive 2001/82/EC and Directive
2001/83/EC, Part III. It certifies that the substance complies with the
EMA Note for Guidance on
minimising the TSE risk. This certificate should be provided when the applicable selected substance
specified in section 5.5 Substance refers to the applicable ingredient role and the 5.2. Origin of the
substance data element is specified.
• The Certificate of Suitability (CEP): Indicate if a Ph. Eur. Certificate of suitability has been issued
for the active substance(s). This certificate certifies that the quality of a given substance is suitably
controlled by the Ph.Eur. monograph with addition of tests if necessary (stated on the CEP).
• Active Substance Master File (ASMF) certification: to indicate if an ASMF has been used for the
active substance(s). This certificate provides detailed information on the manufacturing of the
active substance of a medicine. Depending on the type of certificate obtained (i.e., EU / EMA /
National submission) the relevant reference details shall be provided (i.e., EU/ASMF reference
number shall be specified in case of EU ASMF. The EU ASMF number can be used for Centralised,
Mutual Recognition and Decentralised Procedures; EMEA/ASMF number should be specified only for
ASMFs to be assessed through the Centralised procedure).
• Vaccine Antigen Master File (VAMF) certification: to indicate an EMA certificate for a VAMF has
been issued or submitted in accordance with Directive 2001/83/EC Annex I, Part III.
• Plasma Master File (PMF) certification: this certificate provides detailed information on the
characteristics of the human plasma used in a medicinal product.
The (Certificate) master file class is conditional and repeatable while the individual attributes of this
class are to be repeated and completed as applicable.
(Certificate) master file Class Description
Repeatable
Yes
Conformance
Conditional
5.5.4.1. File type
Tag Description
User Guidance The applicable type of master file should be specified.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/220000000070
Value(s)
As listed in Master File Type RMS list
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
Type
FHIR Path DocumentReference.type (referenced from
Ingredient.extension.masterFile)

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 201/231
Tag Description
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
5.5.4.2. File code
The File code sub-class and it’s attributes are mandatory and not repeatable.
(Certificate) master file Class Description
Repeatable
No
Conformance
Mandatory
5.5.4.2.1. File identifier type
Tag Description
User Guidance
The applicable certificate provided by the relevant Competent Authority
following successful submission shall be specified in PMS.
The file code shall always refer to the current version to the last submitted
version.
Repeatable
No
Conformance
Mandatory
Data Type
CodableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000026028
Value(s)
The applicable value as listed in the Master File Identifier Type RMS list
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
system
FHIR Path DocumentReference.identifier.system (referenced from
Ingredient.extension.masterFile)
Example:
For ASMF: EU ASMF number, National ASMF number, Applicant part version number
5.5.4.2.2. File Identifier
Tag Description
User Guidance
The applicable identifier as assigned by the relevant Competent Authority
following successful submission shall be specified in PMS.
Repeatable
No
Conformance
Mandatory
Data Type
Identifier
RMS URI/URL
Not applicable
Value(s)
The relevant identifier as assigned shall be specified

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 202/231
Tag Description
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
value
FHIR Path DocumentReference.identifier.value (referenced from
Ingredient.extension.masterFile)
5.5.4.3. Submission date
Tag Description
User Guidance The date when the certificate was submitted to the relevant Competent
Authority shall be specified.
Repeatable
No
Conformance Based on the following terms specified in 5.5.4.1:
• Conditional:
− CEP
− ASMF
− VAMPF
− PMF
• Not applicable:
− CEP TSE
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s) A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
submissionDate
FHIR Path DocumentReference.extension.submissionDate
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
5.5.4.4. Date of last update
Tag Description
User Guidance The date when the certificate was updated most recently shall be specified
where applicable.
Repeatable
No
Conformance Based on the following terms specified in 5.5.4.1:
•
Mandatory:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 203/231
Tag Description
− CEP
− ASMF
− VAMPF
− PMF
• Not applicable:
− CEP TSE
Data Type
dateTime
RMS URI/URL
Not applicable
Value(s) A date shall be specified using the ISO 8601 date format.
ISO 8601 can accommodate year and month should day of the month not
be known. (i.e., YYYY-MM).
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name dateOfUpdate
FHIR Path DocumentReference.extension.dateOfUpdate
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
5.5.4.5. Manufacturer
Tag Description
User Guidance
The reference to the relevant Manufacturer of the selected substance shall
be selected from on the list of manufacturers recorded in section 5.4.
Manufacturer.
This data field shall be specified in case of the following certificates type:
CEP, ASMF, VAMF certificates.
Repeatable
Yes
Conformance
Conditional
Data Type
Reference
RMS URI/URL
Not applicable
Value(s) Reference to the relevant ActivityDefinition resource describing the
manufacturing business operation.
ISO Element Name
Not applicable
ISO Path
Not applicable
FHIR Element Name
subject
FHIR Path DocumentReference.extension.manufacturingBusinessOperation
Note: Please refer to Chapter VI – SPOR API Technical Specification for the
details of the extension URL.
6. Pharmaceutical product
Each authorised medicinal product shall contain at least one pharmaceutical product.

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 204/231
The technical concept of a "pharmaceutical product", refers to the qualitative and quantitative
composition of a medicinal product in the pharmaceutical form, approved for administration to the
patient, in line with the regulated product information.
A medicinal product may contain one or more "pharmaceutical product(s)" (e.g., a kit containing
vaginal tablets 500 mg and a vaginal cream 10% or a kit containing a combination of norethindrone
acetate and ethinyl estradiol tablets and ferrous fumarate tablets). In these instances, a
pharmaceutical product section is to be completed for each "pharmaceutical product".
Where applicable, the technical concept of a "pharmaceutical product" can also include information on
a medical device if it is an "integral part" of the medicinal product and supports the
pharmacological/metabolic/immunological action of the medicinal product, for example the scaffolding
or net for a cell therapy medicinal product in accordance with Regulation (EC) No 1394/2007.
The administrable pharmaceutical form refers to the pharmaceutical form for administration to the
patient, after any necessary transformation of the "manufactured" pharmaceutical form has been
carried out. This concept is illustrated in the following examples.
If a product undergoes several transformations before it is given to the patient, intermediate forms
that are not given to the patient will not be captured in the data. Only the final form(s) that are given
to the patient should be included in the pharmaceutical product data.
Example(s):
Example 1:
Medicinal Product ABC 20mg/mL powder and solvent for solution for injection (combined
pharmaceutical form) will contain two types of manufactured items with the following manufactured
dose forms:
• Powder for solution for injection
• Solvent for Solution for injection
In this case the medicinal product needs to undergo the necessary transformation prior to the
administration to the patient and the pharmaceutical form is as follows:
Administrable dose form Solution for Injection
Strength 20mg/mL
Unit of Presentation Vial
Example 2:
Medicinal Product DEF 500 mg tablets contain a single type of manufactured item with the following
manufactured dose form:
• Tablet
In this case the medicinal product does not need to undergo any transformation prior to the
administration to the patient and the administrable dose form equals the manufactured dose form. The
pharmaceutical form is as follows:
Administrable dose form Tablet

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 205/231
Strength 500 mg / unit
Unit of Presentation Tablet
Each pharmaceutical product shall contain information on:
• Active ingredient(s) - active ingredient substance name(s) and its/their amounts(s) can be found in
Module 3.2.P.1 – Description and Composition of the Drug Product. This information is also
reflected in section 2. Qualitative and Quantitative Composition of the corresponding SmPC;
• Excipient(s) - excipient substance name(s) and their amount(s) can be found in Module 3.2.P.1 –
Description and Composition of the Drug Product. This information is also reflected in section 6.1
List of excipients of the corresponding SmPC. In case that Module 3.2.P.1 have additional
granularity on the components of an individual excipient, the information on the SmPC should be
taken as a reference, corresponding with the highest level of the excipient described in 3.2.P.1,
provided this "multi-components Excipient" is registered in SMS.
• In some instances, pharmaceutical product can also contain adjuvants. Adjuvant substance
name(s) and its/their amount(s) can be found in Module 3.2.P.1 – Description and Composition of
the Drug Product. This information is also reflected in section 2. Qualitative and Quantitative
Composition (or in section 6.1 List of excipients) of the corresponding SmPC;
• The ingredient(s) of a manufactured item or of the pharmaceutical product shall be specified based
on the Resource Ingredient as outlined below.
Note: The contents of the document [i.e., Module 1.2 – Electronic Application form (eAF), Relevant
sections in Module 3 – Quality, Summary of Product Characteristics (SmPC)] supporting the regulatory
process shall be aligned, where applicable, to ensure the discrepancies between the documents are
minimized. The content should enhance the quality of the product data reported in Product
Management Service (PMS). This requirement applies to new medicinal products single entry in PMS.
Based on the above principle. the SmPC as authorized/to be authorized is the main referring document
for data entry purposes.
However, for medicinal product entry already available in PMS, following the data load from XEVMPD to
PMS database (existing product data), whenever the common contents of each of the above supporting
documentation are not aligned, the information available in the relevant sections in Module 3 can be
used to harmonize the values in PMS. This requirement applies provided data confidentiality is ensured
and if no additional complexity is added to the data entry in PMS. For additional information, refer to
section 1.3.1 of EU IG Chapter 8 – Practical example.
Note: Further information referring to existing product data will be made available in the EU IG
Chapter 9 - Process for submitting existing data on medicinal products authorised for human use. This
chapter is under development and it will be made available at later stage.
The full information on Pharmaceutical Product as presented in the FHIR Resource
AdministrableProductDefinition is shown below. In the context of the Iteration 1 of the PMS
implementation, the elements included in the red rectangle below are in scope and should be provided
according to the rules and guidance described in this section:

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 206/231
Figure 32: Resource AdministrableProductDefinition (Source: http://www.hl7.org/fhir
)
The Pharmaceutical product class is mandatory and repeatable while the individual attributes of this
class are not repeatable (with the exception of the ingredient and device data elements class) and shall
be populated as applicable.
Pharmaceutical product Class Description
Repeatable
Yes
Conformance
Mandatory
6.1. Pharmaceutical product description
The description of pharmaceutical product shall be provided whenever the medicinal product contains
more than one pharmaceutical product in order to allow differentiation in the database.
The provision of pharmaceutical product description is not needed for medicinal products with a single
pharmaceutical product.
In medicinal products containing more than one pharmaceutical product (e.g., contraceptive having
different strengths and fixed dose combination as part of the same medicinal product), differentiation
of manufacturing items is not easily visualised with the information provided in the rest of individual
data fields (in certain cases it can only be differentiated on specific characteristics of substances
including the qualitative and quantitative composition of the pharmaceutical product).
Information on pharmaceutical product description is free text and should be provided in the form of:
“Physical Characteristics” + “Unit of presentation”.
Physical Characteristics of the pharmaceutical product item includes colour or shape among others
which allow differentiation from remaining pharmaceutical products.
Tag Description
User Guidance The high-level description of pharmaceutical product shall be provided
whenever the medicinal product contains more than one pharmaceutical
product (e.g., different types of tablets in the same package).

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 207/231
Tag Description
Information on pharmaceutical item description is free text and should be
provided in the form of: “Physical Characteristics” + “Unit of presentation”.
Products authorised through MRP/DCP/NP routes
• The pharmaceutical product description is to be provided in English or in
the local language(s) of authorisation, or optionally in all of them.
Products authorised through the centralised procedure
The pharmaceutical product description is to be provided in English.
Repeatable
Yes
Conformance
Conditional
Data Type Markdown
RMS URI/URL
Not applicable
Value(s)
Free text or markdown text for rich content
ISO Element
name
Not Applicable
ISO Path
Not Applicable
FHIR Element
name
Text
FHIR Path
AdministrableProductDefinition.property.valueCodeableConcept.text
Example(s):
Dark yellow film-coated tablet to differentiate from Medium red film-coated tablet
6.1.1. Language
This section described how to populate information related to the language of the pharmaceutical
product description. The provision of the language is mandatory.
Tag Description
User Guidance The language of the pharmaceutical product description, as approved by
the regulatory authority and indicated in the corresponding regulatory
document(s) shall be specified as a term ID.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000072057
Value(s)
As indicated in the Language RMS list
ISO Element Name
Not Applicable
ISO Path
Not Applicable
FHIR Element Name
language
FHIR Path AdministrableProductDefinition.property.valueCodeableConcept.text.extens
ion.language

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 208/231
6.2. Administrable dose form
Tag Description
User Guidance The administrable dose form corresponds with the dose form intended for
administration to the patient, after any necessary transformation of the
manufactured dose form has been carried out. This information shall be
provided in line with the information indicated in Section 3. Pharmaceutical
form of the corresponding SmPC.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000000004
Value(s)
Listed in the Pharmaceutical Dose Form RMS list
ISO Element name
Administrable Dose Form
ISO Path
/MedicinalProduct/PharmaceuticalProduct/AdministrableDoseForm
FHIR Element name
administrableDoseForm
FHIR Path
AdministrableProductDefinition.administrableDoseForm
Example(s):
Tablets, Solution for injection, Capsule
6.3. Unit of presentation
Tag Description
User Guidance The unit of presentation describing the unit in which a "pharmaceutical
product" is administered to describe the strength or quantity. The unit of
presentation shall be specified as a term ID.
The value shall be selected from the term ID as listed in the applicable
Referentials Management Service (RMS) list.
Repeatable
No
Conformance
Conditional
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/200000000014
Value(s)
As listed in the Units of Presentation RMS list
ISO Element name Unit of Presentation
ISO Path
/MedicinalProduct/PharmaceuticalProduct/UnitOfPresentation
FHIR Element name
unitOfPresentation
FHIR Path
AdministrableProductDefinition.unitOfPresentation
Example(s):
- units of presentation: puff, actuation, patch, tablet, vial

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 209/231
6.4. Ingredient
The ingredient(s) of the pharmaceutical product shall be selected from the previously recorded list of
ingredients based on the Resource Ingredient as outlined in section 5. Ingredient
The Ingredient class is mandatory. For further information related to the technical details and business
rules please refer to section 5. Ingredient. This value is an attribute within Pharmaceutical Product
domain.
Tag Description
User Guidance The reference to the ingredient(s) of the pharmaceutical product shall be
selected from the list of ingredients recorded in section 5. Ingredient.
Repeatable
Yes
Conformance
Mandatory
Data Type
Reference
RMS URI/URL
Not applicable
Value(s) Structured data elements referencing the included ingredients in the
manufactured item
ISO Element Name
Ingredient
ISO Path For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Ingredient
FHIR Element name
Ingredient
FHIR Path
AdministrableProductDefinition.ingredient
6.5. Device
A medical device may support the pharmacological/metabolic/immunological action of the medicinal
product (e.g., collagen scaffold). These types of devices are considered part of the pharmaceutical
product administered to the patient and should be recorded as part of the pharmaceutical product.
Any other device co-packaged (e.g., spoon, syringe) or integral (e.g., pre-filled pen) with the medicinal
product shall be recorded as part of the packaged medicinal product with the attributes described in
section 4.10. Medical device.
When applicable, the information shall be provided as described in section 4.10. Medical device. The
applicable value(s) shall be selected from the term ID as listed in the Referentials Management Service
(RMS).
Example(s):
collagen scaffold
Tag Description
User Guidance The reference to the medical device(s) which is part of the administered
pharmaceutical product shall be selected from the list of medical device(s)
recorded in section 4.10 Medical device.
Repeatable
Yes
Conformance
Conditional
Data Type
Reference
RMS URI/URL
Not applicable

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 210/231
Tag Description
Value(s) Structured data element referencing the included device in the
pharmaceutical form
ISO Element Name
Device
ISO Path For the Pharmaceutical product, the ISO path is:
/MedicinalProduct/PharmaceuticalProduct/Device
FHIR Element name
Device
FHIR Path
AdministrableProductDefinition.device
6.6. Route of administration
Tag Description
User Guidance The route of administration of the pharmaceutical form shall be specified in
accordance with Section 4.2. Posology and method of administration of the
SmPC as Term ID.
Administration route section describes the route(s) of administration i.e.,
the path by which the medicinal product (described as technical concept of
a "pharmaceutical product") is taken into or makes contact with the body.
The applicable value shall be selected from the term ID as listed in the
applicable Referentials Management Service (RMS) list.
Repeatable
Yes
Conformance
Mandatory
Data Type
CodeableConcept
RMS URI/URL
https://spor.ema.europa.eu/v1/lists/100000073345
Value(s)
As listed in Routes and Methods of Administration RMS List
ISO Element name
Route of Administration
ISO Path /MedicinalProduct/PharmaceuticalProduct/RouteOfAdministration/RouteOfA
dministration
FHIR Element name
Code
FHIR Path
AdministrableProductDefinition.routeOfAdministration.code
Example(s):
Oral use, intravenous use, oromucosal use, ocular use

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 211/231
7. Annex I - PMS ID, MPIDs and PCIDs relationship during lifecycle of medicinal products –
Examples
This annex provides a description of the relationship and evolution of identifiers from the first submission and during the lifecycle of medicinal products. For
more information, please refer to Introduction Section of Chapter 2: Data elements for the electronic submission of information on medicinal products for
human use.
7.1. MPIDs/PCIDs examples* CAPs
Action
i
Procedure number
ii
Version
iii
Content of
change
Medicinal
product
iv
Packag
e
MAH
name/ID
v
PMS ID
vi
MPID
vii
Marketing
Authorisatio
n number
(Medicinal
Product
Level)
viii
PCID
ix
Marketing
Authorisation
number (Package
Medicinal Product
Level)
x
Submissio
n
EMA/H/C/000123/0000 v1 Initial
submission;
MPID is
generated
Product A,
50mg,
<active
substance>
, Tablet,
MAH A, EU
Blister
(alu) 1
tablet
Blister
(alu) 5
tablets
MAH A/
100000396
0001265
4
EU-
100000396-
00010000
EU/1/98/077
(root number)
EU-
100000396-
00010000-
0001
EU-
100000396-
00010000-
0002
EU/1/98/077/001
EU/1/98/077/002
Type 1A
variation
EMA/H/C/000123/IA/000
1
v2 change of
admin data
no change no
change
no change no
change
no change no change no change no change
Type II
variation
EMA/H/C/000123/II/0002 v3 change of
manufacture
r
no change no
change
no change no
change
no change no change no change no change

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 212/231
Action
i
Procedure number
ii
Version
iii
Content of
change
Medicinal
product
iv
Packag
e
MAH
name/ID
v
PMS ID
vi
MPID
vii
Marketing
Authorisatio
n number
(Medicinal
Product
Level)
viii
PCID
ix
Marketing
Authorisation
number (Package
Medicinal Product
Level)
x
Transfer EMA/H/C/000123/T/0003 v4 change of
MAH
Product A,
50mg,
<active
substance>
, Tablet,
MAH B, EU
no
change
MAH B/
10000095
5
no
change
EU-
100000955
-00010000
no change EU-
100000955
-00010000-
0001
EU-
100000955
-00010000-
0002
no change
Type 1A
variation
EMA/H/C/000123/IA/000
4
v5 change of
admin data
no change no
change
no change no
change
no change no change no change no change
Extension
of
therapeuti
c
indication
EMA/H/C/000123/II/0005 v6 Introduction
of new
therapeutic
indication
no change no
change
no change no
change
EU-
100000955-
00020046
no change EU-
100000955-
00020046-
0001
EU-
100000955-
00020046-
0002
no change
Type 1A
variation
EMA/H/C/000123/IA/000
6
v7 change of
admin data
no change no
change
no change no
change
no change no change no change no change
Transfer EMA/H/C/000123/T/0007 v8 change of
MAH
Product A,
50mg,
<active
no
change
MAH C/
10001407
8
no
change
EU-
100014078
-00020046
no change EU-
100014078
-00020046-
no change

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 213/231
Action
i
Procedure number
ii
Version
iii
Content of
change
Medicinal
product
iv
Packag
e
MAH
name/ID
v
PMS ID
vi
MPID
vii
Marketing
Authorisatio
n number
(Medicinal
Product
Level)
viii
PCID
ix
Marketing
Authorisation
number (Package
Medicinal Product
Level)
x
substance>
, Tablet,
MAH C, EU
0001
EU-
100014078
-00020046-
0002
Type II
variation
EMA/H/C/000123/II/0008 v9 Change of
legal status
Product A,
50mg,
<active
substance>
, Tablet,
MAH C, EU
no
change
no change no
change
no change no change no change no change
Type IB
variation
EMA/H/C/000123/IB/000
9
v10 Addition of
pack size
Product A,
50mg,
<active
substance>
, Tablet,
MAH C, EU
Blister
(alu) 1
tablet
Blister
(alu) 5
tablets
Blister
(alu) 20
tablets
no change no
change
no change no change EU-
100014078-
00020046-
0001
EU-
100014078-
00020046-
0002
EU-
100014078
-00020046-
0003
EU/1/98/077/001
EU/1/98/077/002
EU/1/98/077/00
3
Type 1A
variation
EMA/H/C/000123/IA/001
0
v11 change of
admin data
Product A,
50mg,
no
change
no change no
change
no change no change no change no change
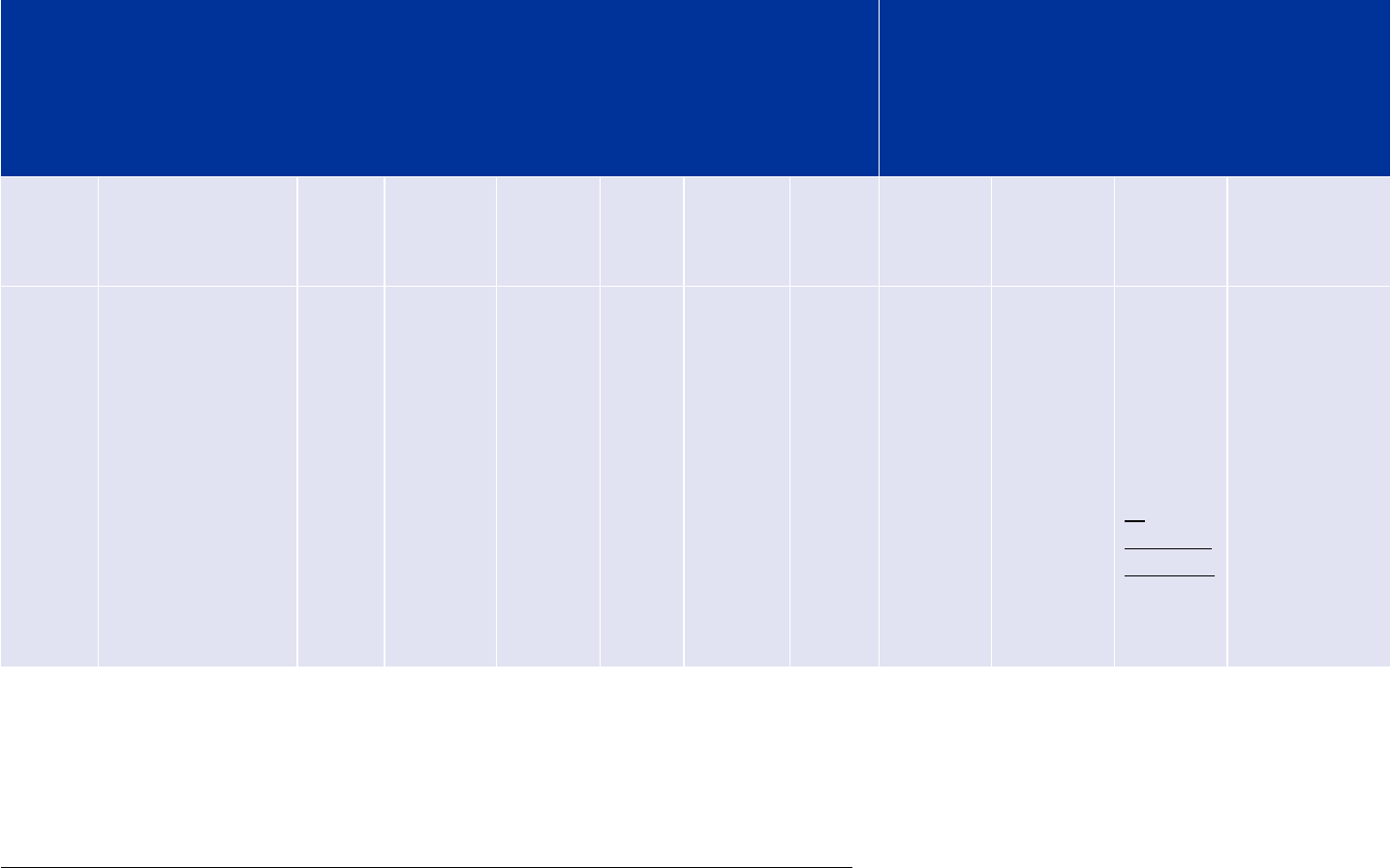
Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 214/231
Action
i
Procedure number
ii
Version
iii
Content of
change
Medicinal
product
iv
Packag
e
MAH
name/ID
v
PMS ID
vi
MPID
vii
Marketing
Authorisatio
n number
(Medicinal
Product
Level)
viii
PCID
ix
Marketing
Authorisation
number (Package
Medicinal Product
Level)
x
<active
substance>
, Tablet,
MAH C, EU
Type IB
variation
EMA/H/C/000123/II/0011 v10 change of
packaging
Product A,
50mg,
<active
substance>
, Tablet,
MAH C, EU
Blister
(alu) 1
tablet
Blister
(alu) 5
tablets
Bottle
(glass)
20
tablets
no change no
change
no change no change EU-
100014078-
00020046-
0001
EU-
100014078-
00020046-
0002
EU
100014078
-0002-0003
EU-
100014078
-0002-0004
no change

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 215/231
7.2. MPIDs/PCIDs examples* MRP/DCP
Action
i
Procedure
number
ii
Version
iii
Content Medicinal
product
iv
Package MAH
name/ID
v
PMS ID
vi
MPID
vii
Marketing
Authorisation
number
(Medicinal
Product
Level)
viii
PCID
ix
Marketing
Authorisation
number
(Package
Medicinal
Product Level)
x
Submission in
Sweden
FR/H/0121/03
v1
initial
submission
via MRP;
MPID is
generated
Product S, 120
mg, <active
substance>,
film-coated
tablet, MAH D,
SE
Blister
(plast,
alu) 100
tablets
Blister
(plast,
alu) 200
tablets
MAH D/
100000999
(SE)
00001997
SE-
100000999-
00000001
13650
SE-
100000999-
00000001-
0001
SE-
100000999-
00000001-
0002
Not applicable -
Blank
Type I
variation
FR/H/0121/03
v2
change of
shelf life
no change
no change
no change
no
change
no change
no change
no change
Not applicable -
Blank
Type II
variation
FR/H/0121/03
v3
change of
SmPC,
change in
section 4.8
(adverse
reactions)
no change
no change
no change
no
change
no change
no change
no change
Not applicable -
Blank
Transfer
FR/H/0121/03
v4
change of
MAH
Product S, 120
mg, <active
substance>,
film-coated
tablet, MAH
E, SE
no change
MAH E /
100001563
(SE)
no
change
SE-
100001563-
00000001
no change
SE-
100001563-
00000001-
0001
SE-
100001563-
00000001-
0002
Not applicable -
Blank
Type IA
variation
FR/H/0121/03
v5
change of
admin data
no change
no change
no change
no
change
no change
no change
no change
Not applicable -
Blank
Type IB
variation
FR/H/0121/03
v6
Change of
product
name
Product S01,
120 mg,
<active
substance>,
film-coated
no change
no change
no
change
SE-
100001563-
00000002
no change
SE-
100001563-
00000002-
0001
SE-
100001563-
Not applicable -
Blank

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 216/231
Action
i
Procedure
number
ii
Version
iii
Content
Medicinal
product
iv
Package
MAH
name/ID
v
PMS ID
vi
MPID
vii
Marketing
Authorisation
number
(Medicinal
Product
Level)
viii
PCID
ix
Marketing
Authorisation
number
(Package
Medicinal
Product Level)
x
tablet, MAH E,
SE
00000002-
0002
Type II
variation
FR/H/0121/03
v7
Any change
to indication
no change
no change
no change
no
change
SE-
100001563-
00000003
no change
SE-
100001563-
00000003-
0001
SE-
100001563-
00000003-
0002
Not applicable -
Blank
Type IA
variation
FR/H/0121/03
v8
Change of
admin data
no change
no change
no change
no
change
no change
no change
no change
Not applicable -
Blank
Transfer
FR/H/0121/03
v9
Change of
MAH
Product S01,
120 mg,
<active
substance>,
film-coated
tablet, MAH
G, SE
no change
MAH G /
100001745
(SE)
change
no
SE-
100001745-
00000003
no change
SE-
100001745-
0000003-
0001
SE-
100001745-
00000003-
0002
Not applicable -
Blank
Change of
legal status
FR/H/0121/03
v10
Change of
legal status
no change
Blister
(plast,
alu) 100
tablets
Blister
(plast,
alu) 200
tablets
Blister
(plast,
alu) 10
tablets
(OTC)
no change
no
change
no change
no change
SE-
100001745-
0000-003-
0001
SE-
100001745-
00000003-
0002
SE-
100001745-
00000003-
0003
Not applicable -
Blank

Product Management Service (PMS) - Implementation of International Organization for
Standardization (ISO) standards for the identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 217/231
Action
i
Procedure
number
ii
Version
iii
Content
Medicinal
product
iv
Package
MAH
name/ID
v
PMS ID
vi
MPID
vii
Marketing
Authorisation
number
(Medicinal
Product
Level)
viii
PCID
ix
Marketing
Authorisation
number
(Package
Medicinal
Product Level)
x
Administrative
change
IT/H/0805/02
v11
Change of
RMS
no change
no change
no change
no
change
no change
no change
no change
Not applicable -
Blank

Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands
An agency of the European Union
Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us
Send us a question Go to www.ema.europa.eu/contact
Telephone +31 (0)88 781 6000
© European Medicines Agency, 2022. Reproduction is authorised provided the source is acknowledged.
8. Annex II - Common/European Union (EU) and national data set
This section provides a comprehensive list of the different resources, classes and related attributes defined in the Product Management Service (PMS)
database. The below tables illustrate how the different data elements and related product data values apply based on the different EU regulatory
authorization procedural context.
Term Centralised Procedure (CAP) context
Mutual Recognition Procedure (MRP) /Decentralized Procedure (DCP)
context
European
Union (EU)
The same value(s) is applicable across all EU Member
States. The value shall not differ across the EU Member
States.
This term is applicable to medicinal products
authorised/to be authorised under Centralised
Procedure(s).
This term is not applicable to medicinal products authorised/to be authorised
under non-Centralised Procedure(s).
The term Common shall apply.
Common This term is not applicable to medicinal products
authorised/to be authorised under Centralised
Procedure(s).
The term European Union shall apply.
The same value(s) is applicable across all Member State(s) related to the
specific non-CAP regulatory authorisation procedure (MRP/DCP).
This term is applicable to medicinal products authorised/to be authorised under
non-Centralised Procedure(s).
National The different value(s) based on the National requirements
is applicable to each individual Member State(s).
The different value based on the National requirements is applicable to each
individual Member State(s) related to the specific non-CAP regulatory
authorisation procedure (MRP/DCP) National procedure.
EU &
National
At least one (or the) common value(s) is applicable across
the EU Member States. Additionally, based on National
legal requirement a separate value shall also apply.
Based on EEA agreements a separate value shall apply to
Iceland, Liechtenstein and Norway [Article 57(2) of
Regulation (EC) No. 726/2004)].
This term is not applicable to medicinal products authorised/to be authorised
under non-Centralised Procedure(s).
This term Common & National shall apply.

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 219/231
This term is applicable to medicinal products
authorised/to be authorised under Centralised
Procedure(s).
Common &
National
This term is not applicable to medicinal products
authorised/to be authorised under Centralised
Procedure(s).
The term EU & National shall apply.
At least one (or the) common value(s) is applicable across the EU Member
States involved in the specific non-CAP regulatory authorisation procedure
(MRP/DCP). Additionally, based on National legal requirement a separate value
shall also apply.
This term is applicable to medicinal products authorised/to be authorised under
non-Centralised Procedure(s).
Legend for repeatable fields:
≠ - Referring to repeatable data attribute as a class
‡ - Referring to repeatable data attribute as a data element
Additional demarcations
* - Marking linked classes of data elements
• - Marking data elements subjects to RMS lists
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
1
0
0
Provenance
•
Mandatory
-
-
2
0
Reason•
Mandatory
Not applicable (Technical)
Not applicable (Technical)
3
0
Target
Mandatory
Not applicable (Technical)
Not applicable (Technical)
4
0
Recorded
Mandatory
Not applicable (Technical)
Not applicable (Technical)
5
0
Sender
Mandatory
Not applicable (Technical)
Not applicable (Technical)
6
1
1.
Medicinal product
Mandatory
-
-

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 220/231
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance
Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
7 1.1. Product Management Service
Identifier (PMS ID)
Not
applicable/Ma
ndatory
EU & National
(IS, LI, NO)
6
(Dependent on the respective
regulatory authorisation type)
8 1.2. Medicinal Product Identifier (MPID) Not applicable EU & National
(IS, LI, NO)
7
(Dependent on the respective
regulatory authorisation type)
9
1.3.
Domain
•
Mandatory
EU
Common
10
1.4.
Type
•
Mandatory
EU
Common
11 1.5. (Authorised) pharmaceutical
form
•
‡
Mandatory EU Common
12 1.6. Combined pharmaceutical dose
form
•
Conditional EU Common
13
1.7.
Legal status of supply
•
Mandatory
EU
Common
14 1.8. Additional monitoring indicator Mandatory EU Common
15
1.9.
Orphan Designation
8≠
Conditional
-
-
16
1.9.1.
Regulatory authorisation type
•
Mandatory
EU
Common & National
17
1.9.2.
Orphan designation status
•
Mandatory
EU
Not applicable
18
1.9.3.
Orphan designation number
Mandatory
EU
Not applicable
19
1.9.4.
Orphan designation status date
Mandatory
EU
Not applicable
20
1.9.5.
Market exclusivity start date
Mandatory
EU
Not applicable
21
1.10.
Paediatric use indicator
Mandatory
EU
Common & National
22 1.11. Full indication text
‡
Mandatory/
Optional
EU & National National
23
1.11.1.
Language
•
Mandatory
EU & National
National
24
1.12.
EURD ID
Conditional
EU
Common
6
Please refer to the section 1.1. PMS ID of this Chapter for further details. When referring to “EU & National” we are referring to the “country” entities European Union (EU, IS, LI, NO.
7
Please refer to the section 1.2 MPID of this Chapter for further details. When referring to “EU & National” we are referring to the “country” entities European Union (EU, IS, LI, NO.
8
Orphan designation only applicable to EU as authorizations for IS, LI and NO are national authorisations from the corresponding regulatory authorities.

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 221/231
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance
Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
25
1.13.
Product classification
Mandatory
-
-
26
1.13.1.
xEVMPD product type information
•
Mandatory
EU
Common
27
1.13.2.
Legal basis
•
Mandatory
EU
Common
28
1.13.3.
ATC code(s)
•
Mandatory
EU
Common
29
1.13.3.1.
ATC code(s) - Flag
Conditional
EU
Common
30
1.13.4.
Medicinal product category
•
‡
Mandatory
EU
Common
31 1.13.5. Genetically Modified Organisms
(GMOs)
Mandatory EU Common
32
1.14.
Medicinal product name
≠
Mandatory
-
-
33
1.14.1.
Full name
‡
Mandatory
EU & National
National
34
1.14.2.
Country/Language
Mandatory
EU & National
National
35
1.14.2.1.
Country
•
Mandatory
EU & National
National
36
1.14.2.2.
Language
•
Mandatory
EU & National
National
37 1.14.3. (Medicinal product name) name
part(s)
≠
Mandatory EU & National Common & National
38
1.14.3.1.
Name part type
•
Mandatory
EU & National
Common
39
1.14.3.2.
Name part text
‡
Mandatory
EU & National
Common & National
40
1.14.3.3.1.
Invented name part
Conditional
EU & National
Common
41
1.14.3.3.2.
Scientific part
Conditional
EU & National
National
42
1.14.3.3.3.
Strength part
Conditional
EU & National
Common
43
1.14.3.3.4.
Pharmaceutical dose form part
Conditional
EU & National
National
44 1.14.3.3.5. Formulation part Conditional EU & National National
45
1.14.3.3.6.
Intended use part
Conditional
EU & National
National
46
1.14.3.3.7.
Target population part
Conditional
EU & National
National
47
1.14.3.3.8.
Container or pack part
Conditional
EU & National
National
48
1.14.3.3.9.
Device part
Conditional
EU & National
National

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 222/231
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance
Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
49
1.14.3.3.10.
Trademark or company name part
Conditional
EU & National
National
50
1.14.3.3.11.
Time/period part
Conditional
EU & National
National
51
1.14.3.3.12.
Flavour part
Conditional
EU & National
National
52
1.14.3.3.13.
Delimiter part
Conditional
EU & National
National
53 1.15. (Pharmacovigilance) master
file
Mandatory - -
54
1.15.1.
File type
•
Mandatory
EU
Common
55
1.15.2.
File code
Mandatory
EU
Common
56
1.16.
Contact (QPPV)
Mandatory
-
-
57
1.16.1.
Identifier
Mandatory
EU
Common
58
1.16.2.
Role
•
Mandatory
EU
Common
59 1.17. Pharmacovigilance enquiry
information
≠
Mandatory - -
60
1.17.1.
Email address
Mandatory
EU
Common
61
1.17.2.
Phone number
Mandatory
EU
Common
62
1.17.3.
Role
•
Mandatory
EU
Common
63
1.18.
Attached document
≠
Mandatory
-
-
64 1.18.1. Master (Attached document)
Identifier
Conditional EU National
65
1.18.1.1.
Identifier value
Mandatory
EU
National
66
1.18.1.2
Identifier system
•
Mandatory
EU
National
67 1.18.2. Alternative (Attached document)
Identifier‡
Optional EU & National National
68
1.18.2.1.
Identifier value
Mandatory
EU & National
National
69
1.18.2.2.
Identifier system
•
Mandatory
EU & National
National
70
1.18.3.
(Attached document) Type
•
Mandatory
EU & National
National

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 223/231
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance
Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
71 1.18.4. (Attached document) Effective
date
Conditional EU & National National
72
1.18.5.
(Attached document) Language
•
≠
Mandatory
EU & National
National
73
1.18.6.
URL value
Mandatory
EU & National
National
74
1.18.7
(Attached document) Status
Mandatory
EU & National
National
75
1.19.
Product cross-reference
≠
Conditional
-
-
76
1.19.1.
Product cross-reference type
•
Mandatory
EU & National
Common
77 1.19.2. Product cross-reference resource
identifier
Mandatory EU & National Common
78 1.20. Manufacturing business
operation
≠
Mandatory - -
79
1.20.1.
Manufacturer*
Mandatory
EU
Common & National
80
1.20.2.
Operation type
•
Mandatory
EU
Common & National
81
1.20.3.
Manufacturing operation start date
Conditional
EU
Common & National
82
1.20.4.
Manufacturing operation end date
Conditional
EU
Common & National
83
1.20.5.
Confidentiality indicator
•
Mandatory
EU
Common & National
84 1.20.6. Manufacturing authorisation
reference number
Conditional EU Common & National
85
1.20.7.
Effective date
Conditional
EU
Common & National
86 1.20.8. (Manufacturing business
operation) Medicines Regulatory
Agency Organisation
Mandatory
(ORG ID);
Conditional
(LOC ID)
EU Common & National
87 2 2. Marketing authorisation
information
Mandatory - -
88
2.1.
Regulatory authorisation type
•
Mandatory
EU
Common
89
2.2.
Marketing authorisation number
Conditional
EU
National

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 224/231
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance
Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
90 2.3. Country
•
Mandatory EU & National
(IS, LI, NO)
National
91 2.4. Authorisation status
•
Mandatory EU & National
(IS, LI, NO)
National
92 2.5. Authorisation status date Conditional EU & National
(IS, LI, NO)
National
93 2.6. Date of first authorisation Mandatory EU & National
(IS, LI, NO)
National
94
2.7.
International birth date
Mandatory
EU
Common
95 2.8. Marketing authorisation holder
(organisation)
Mandatory EU National
96 2.9. (Marketing authorisation)
Regulator
Mandatory/
Conditional
EU National
97 2.10. Marketing authorisation
procedure
9
Mandatory/
Optional
10
*
- -
98
2.10.1.
Procedure Identifier
Conditional
EU
Common
99 2.10.2. Procedure type – Medicines
approval system
•
Mandatory EU Common
100
2.10.3.
Procedure start date
Conditional
EU
Common
101
2.10.4.
Procedure end date
Conditional
EU
Common
102
2.10.5.
Regulatory application
Conditional
EU
Common & National
103 2.10.5.1. Regulatory application
identifier/Number
Conditional EU Common & National
104
2.10.5.2.
Regulatory application type
•
Mandatory
EU
Common & National
9
Please refer to the section 2.10 in this Chapter for further details. When referring to “EU & National” we are referring to the “country” entities European Union (EU, IS, LI, NO.
10
“Mandatory” conformance designated for medicinal products authorized in EU/EEA and “Optional” for medicinal products outside this region. For further details please refer to the
corresponding section in Chapter 2 for further details.

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 225/231
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance
Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
105
2.10.5.3.
Regulatory application end date
Conditional
EU
Common & National
106 3 3. Therapeutic (product)
indication
≠
Mandatory - -
107 3.1.
Indication as
"Disease/Symptom/Procedure"•
Mandatory EU Common & National
108
3.2.
Co-morbidity
‡
•
Conditional
EU
Common & National
109
3.3.
Intended effect
‡
•
Mandatory
EU
Common & National
110
4
4.
Packaged medicinal product
≠
Mandatory
-
-
111 4.1. Packaged medicinal product
Identifier (PCID)
Not applicable EU & National National
112
4.2.
Package description
‡
Mandatory
EU
National
113
4.2.1.
Language
•
Mandatory
EU & National
National
114
4.3.
Manufacturer‡
Conditional
EU
Common & National
115
4.4
Pack size
‡
Mandatory
EU
National
116
4.4.1.
Quantity operator
•
Mandatory
EU
National
117
4.5.
Legal status of supply
•
Conditional
EU
National
118
4.6.
Marketing status
≠
Mandatory
-
-
119
4.6.1.
Country
•
Mandatory
National
National
120
4.6.2.
Marketing status
•
Mandatory
National
National
121
4.6.3.
(Marketing status) start date
Conditional
National
National
122
4.6.4.
(Marketing status) end date
Conditional
National
National
123
4.6.5.
Risk of supply shortage
Conditional
National
National
124
4.6.6.
Risk of supply shortage comment
Conditional
National
National
125
4.6.7.
Status Reason
Conditional
National
National
126
4.6.7.1
Reason
•
Mandatory
National
National
127
4.6.7.2.
Restore date
Optional
National
National

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 226/231
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance
Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
128 4.7. Marketing authorisation
(Package level)
Mandatory - -
129
4.7.1.
Regulatory authorisation type
•
Conditional
EU
Common & National
130 4.7.2. Marketing authorisation number
(Package level)
Conditional EU National
131
4.7.3.
Country
•
Mandatory
EU
National
132
4.7.4.
Authorisation status
•
Mandatory
EU
National
133 4.7.5. Authorisation status date (Package
level)
Conditional EU National
134
4.8.
Package item (container)
Mandatory
-
-
135
4.8.1.
Package item (container) type
•
Mandatory
EU
Common & National
136
4.8.2.
Package item reference(s)
‡
Conditional
EU
Common & National
137
4.8.3.
Manufactured item reference(s)
‡
Conditional
EU
Common & National
138
4.8.4.
Device reference(s)
‡
Conditional
EU
Common & National
139
4.8.5.
Package item (container) quantity
Conditional
EU
Common & National
140
4.8.5.1.
Quantity operator
•
Mandatory
EU
Common & National
141
4.8.6.
Data carrier identifier
≠
Optional
National
National
142
4.8.6.1.
Identifier value
Mandatory
National
National
143 4.8.6.2. Identifier system
•
Mandatory National National
144
4.8.7.
Material
•
‡
Mandatory
EU
Common & National
145
4.9.
Package (component)
≠
Mandatory
-
-
146
4.9.1.
Component type
•
Mandatory
EU
Common & National
147
4.9.2.
Component material
‡
Conditional
EU
Common & National
148
4.10.
Medical Device*
≠
Conditional
-
-
149 4.10.1. Type of combination product –
Drug/ Device; Biological/ Device
•
Mandatory EU Common & National

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 227/231
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance
Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
150
4.10.2.
Medical Device type
•
Mandatory
EU
Common & National
151
4.10.3.
Medical Device identifier
Conditional
EU
Common & National
152
4.10.4.
Medical Device trade name
Conditional
EU & National
Common & National
153 4.10.5. Medical Device quantity Mandatory/No
t applicable
EU Common & National
154
4.10.5.1
Quantity operator
•
Mandatory
EU
Common & National
155
4.10.6.
Medical device description≠
Mandatory
EU
Common & National
156
4.10.6.1
Language
•
Mandatory
EU
Common & National
157 4.10.7. Medical device description of
intended purpose≠
Mandatory EU Common & National
158
4.10.7.1.
Language
•
Mandatory
EU
Common & National
159 4.10.8. Medical device classification
•
Mandatory/No
t applicable
EU Common & National
4.10.9.
Medical device manufacturer‡
Mandatory
EU
Common & National
160
4.11.
Manufactured item
≠
Mandatory
-
-
161
4.11.1.
Unit of presentation
•
Mandatory
EU
Common & National
162
4.11.2.
Manufactured item quantity
Conditional
EU
Common & National
163
4.11.2.1.
Quantity operator
•
Mandatory
EU
Common & National
164 4.11.3. Manufactured dose form
•
Mandatory EU Common & National
165
4.11.4.
Ingredient*
≠
Mandatory
EU
Common & National
166
4.11.5.
Manufactured item description
‡
Conditional
EU
Common & National
167
4.11.5.1.
Language
•
Mandatory
EU & National
National
168
4.12.
Shelf life/ Storage
≠
Conditional
EU
Common & National
169
4.12.1.
Shelf life type
•
Mandatory
EU
Common & National
170
4.12.2.
Shelf life time period and units
•
Mandatory
EU
Common & National
171
4.12.3.
Special precautions for storage
‡•
Mandatory
EU
Common & National

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 228/231
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance
Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
172
5
5.
Ingredient*
≠
Mandatory
-
-
173
5.1.
Ingredient role
•
Mandatory
EU
Common & National
174
5.2
Origin of the substance
•
Optional
EU
Common
175
5.3.
Composition grouping description
Optional
EU
Common
176
5.4.
Manufacturer*
‡
Conditional
EU
National
177
5.5.
Substance
Mandatory
-
-
178
5.5.1.
Substance
Mandatory
EU
Common & National
179 5.5.2. Substance strength
(quantitative composition)
Conditional EU Common & National
180
5.5.2.1.
Quantity operator
•
Not applicable
EU
Common & National
181
5.5.2.2.
Strength (Presentation)
Conditional
EU
Common & National
182
5.5.2.2.1.
Quantity operator
•
Mandatory
EU
Common & National
183 5.5.2.2.2. Strength (Presentation single
value or low limit) •
Mandatory EU Common & National
184 5.5.2.2.3. Strength (Presentation high limit)
•
Conditional EU Common & National
185
5.5.2.3.
Strength (Concentration)
Conditional
EU
Common & National
186
5.5.2.3.1.
Quantity operator
•
Mandatory
EU
Common & National
187 5.5.2.3.2. Strength (Concentration single
value or low limit) •
Mandatory EU Common & National
188 5.5.2.3.3. Strength (Concentration high
limit) •
Conditional EU Common & National
189 5.5.3. Substance reference strength
(quantitative composition)
≠
Conditional - -
190
5.5.3.1.
Reference substance
Mandatory
EU
Common & National
191
5.5.3.2.
Quantity operator
•
Not applicable
EU
Common & National

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 229/231
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance
Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
192
5.5.3.3.
Reference strength (Presentation)
Conditional
EU
Common & National
193
5.5.3.3.1.
Quantity operator
•
Mandatory
EU
Common & National
194 5.5.3.3.2. Reference strength (Presentation
single value or low limit)•
Mandatory EU Common & National
195 5.5.3.3.3. Reference strength (Presentation
high limit)
Conditional EU Common & National
196 5.5.3.4. Reference strength
(Concentration)
Conditional EU Common & National
197
5.5.3.4.1.
Quantity operator
•
Mandatory
EU
Common & National
198 5.5.3.4.2. Reference strength (Concentration
single value or low limit)•
Mandatory EU Common & National
199 5.5.3.4.3. Reference strength (Concentration
high limit)•
Conditional EU Common & National
200
5.5.4.
(Certificate) master file
Conditional
-
-
201
5.5.4.1.
File type
•
Mandatory
EU
Common
202
5.5.4.2
File code
Mandatory
EU
Common
203
5.5.4.2.1.
File identifier type
•
Mandatory
EU
Common
204
5.5.4.2.2.
File identifier
Mandatory
EU
Common
205 5.5.4.3 Submission date Mandatory/No
t applicable
11
EU Common
206 5.5.4.4 Date of last update Conditional/
Not applicable
EU Common
207
5.5.4.5.
Manufacturer
Conditional
EU
Common
208
6
6.
Pharmaceutical product
≠
Mandatory
-
-
11
“Conditional” conformance designated for any certificate as listed in the section 5.5.4. (Certificate) master file with the exception of the TSE certificate to which “Not applicable”
conformance applies. For further details please refer to the corresponding section in Chapter 2 for further details.

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 230/231
Item
Resourc
e
Definitio
n Level
PMS EU IG
Ref.
PMS data element name Conformance
Applicable values in the
context of CAPs: EU or EU &
National
Applicable values in the
context of non-CAPs (MRPs,
DCPs, NPs): Common when
across all MS or National
209 6.1. Pharmaceutical product
description
‡
Conditional EU Common & National
210
6.1.1.
Language
•
Mandatory
EU & National
National
211
6.2.
Administrable dose form
•
Mandatory
EU
Common & National
212
6.3.
Unit of presentation
•
Conditional
EU
Common & National
213
6.4.
Ingredient*
≠
Mandatory
EU
Common & National
214
6.5.
Device*
≠
Conditional
EU
Common & National
215
6.6.
Route of administration
≠
•
Mandatory
EU
Common & National
i
Action refers to the type of change (usually as a consequence of a regulatory procedure) that results in an update on the product entry in Product Management System
(PMS).
ii
Procedure number refers to the number as given by the Regulator (NCA, EMA) at the time of submission of the regulatory procedure. This information is included as per
section 2.10.5 -
Regulatory application procedure of this document.
iii
Version refers to the number given automatically by the system for each record submitted in Product Management System (PMS).
iv
Medicinal product defined as in the Introduction of this document: Product name, Strength, active substance, pharmaceutical form and country.
v
MAH name/ID refers to the Marketing Authorisation Holder and the ORG ID associated with it in the Organisations Management System (OMS).
vi
PMS ID refers to the system generated ID uniquely assigned to a Medicinal Product level at the time of the first submission of the medicinal product information in
Product Management System (PMS). This ID remains unchanged during the lifecycle of the product.
vii
MPID refers to Medicinal Product Identifier as defined by ISO IDMP. This is further detailed in section Identifiers and defining characteristics of a medicinal product entry
in PMS of this document.

Product Management Service (PMS) - Implementation of International Organization for Standardization (ISO) standards for the
identification of medicinal products (IDMP) in Europe
EMA/285848/2020
Page 231/231
viii
Marketing Authorisation number (Medicinal Product level) refers to the authorisation number or equivalent identifier as granted by the European Commission or
relevant Competent Authorities (Regulator) at the level of the Medicinal Product. In countries where MA numbers are assigned at Packaged Medicinal product level with a
common root number across packages, the root number of the MAA must be provided. In countries where MA numbers are assigned at Package Medicinal product level with
no common root number across packages, this field is to be left blank. This information is included as per section 2.2 – Marketing Authorisation Number.
ix
PCID refers to Packaged Medicinal Product as defined by ISO IDMP. This is further detailed in section Identifiers and defining characteristics of a medicinal product entry
in PMS of this document
.
x
Marketing Authorisation number (Package Medicinal Product level) refers to the authorisation number or equivalent identifier as granted by the European
Commission or relevant Competent Authorities (Regulator) at the level of the Package Medicinal Product. This information is included as per section 4.7.2. – Marketing
Authorisation Number.

